Who needs microtubules? Myogenic reorganization of MTOC, Golgi complex and ER exit sites persists despite lack of normal microtubule tracks
- PMID: 22216166
- PMCID: PMC3246457
- DOI: 10.1371/journal.pone.0029057
Who needs microtubules? Myogenic reorganization of MTOC, Golgi complex and ER exit sites persists despite lack of normal microtubule tracks
Abstract
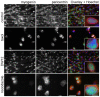
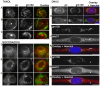
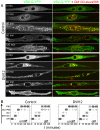
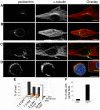
A wave of structural reorganization involving centrosomes, microtubules, Golgi complex and ER exit sites takes place early during skeletal muscle differentiation and completely remodels the secretory pathway. The mechanism of these changes and their functional implications are still poorly understood, in large part because all changes occur seemingly simultaneously. In an effort to uncouple the reorganizations, we have used taxol, nocodazole, and the specific GSK3-β inhibitor DW12, to disrupt the dynamic microtubule network of differentiating cultures of the mouse skeletal muscle cell line C2. Despite strong effects on microtubules, cell shape and cell fusion, none of the treatments prevented early differentiation. Redistribution of centrosomal proteins, conditional on differentiation, was in fact increased by taxol and nocodazole and normal in DW12. Redistributions of Golgi complex and ER exit sites were incomplete but remained tightly linked under all circumstances, and conditional on centrosomal reorganization. We were therefore able to uncouple microtubule reorganization from the other events and to determine that centrosomal proteins lead the reorganization hierarchy. In addition, we have gained new insight into structural and functional aspects of the reorganization of microtubule nucleation during myogenesis.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

