Sensitivity of global translation to mTOR inhibition in REN cells depends on the equilibrium between eIF4E and 4E-BP1
- PMID: 22216185
- PMCID: PMC3245250
- DOI: 10.1371/journal.pone.0029136
Sensitivity of global translation to mTOR inhibition in REN cells depends on the equilibrium between eIF4E and 4E-BP1
Abstract
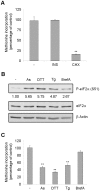
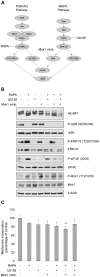
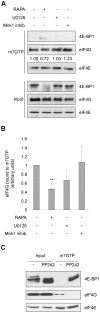
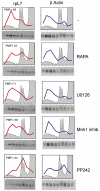
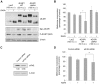
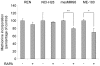
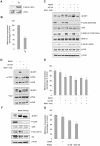
Initiation is the rate-limiting phase of protein synthesis, controlled by signaling pathways regulating the phosphorylation of translation factors. Initiation has three steps, 43S, 48S and 80S formation. 43S formation is repressed by eIF2α phosphorylation. The subsequent steps, 48S and 80S formation are enabled by growth factors. 48S relies on eIF4E-mediated assembly of eIF4F complex; 4E-BPs competitively displace eIF4E from eIF4F. Two pathways control eIF4F: 1) mTORc1 phosphorylates and inactivates 4E-BPs, leading to eIF4F formation; 2) the Ras-Mnk cascade phosphorylates eIF4E. We show that REN and NCI-H28 mesothelioma cells have constitutive activation of both pathways and maximal translation rate, in the absence of exogenous growth factors. Translation is rapidly abrogated by phosphorylation of eIF2α. Surprisingly, pharmacological inhibition of mTORc1 leads to the complete dephosphorylation of downstream targets, without changes in methionine incorporation. In addition, the combined administration of mTORc1 and MAPK/Mnk inhibitors has no additive effect. The inhibition of both mTORc1 and mTORc2 does not affect the metabolic rate. In spite of this, mTORc1 inhibition reduces eIF4F complex formation, and depresses translocation of TOP mRNAs on polysomes. Downregulation of eIF4E and overexpression of 4E-BP1 induce rapamycin sensitivity, suggesting that disruption of eIF4F complex, due to eIF4E modulation, competes with its recycling to ribosomes. These data suggest the existence of a dynamic equilibrium in which eIF4F is not essential for all mRNAs and is not displaced from translated mRNAs, before recycling to the next.
© 2011 Grosso et al.
Conflict of interest statement
Figures
References
-
- Proud CG. eIF2 and the control of cell physiology. Semin Cell Dev Biol. 2005;16(1):3–12. - PubMed
-
- Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell. 2010;40(2):228–237. - PubMed
-
- Hinnebusch AG. Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol. 2005;59:407–450. - PubMed

