The promigratory activity of the matricellular protein galectin-3 depends on the activation of PI-3 kinase
- PMID: 22216245
- PMCID: PMC3247242
- DOI: 10.1371/journal.pone.0029313
The promigratory activity of the matricellular protein galectin-3 depends on the activation of PI-3 kinase
Abstract
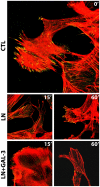
Expression of galectin-3 is associated with sarcoma progression, invasion and metastasis. Here we determined the role of extracellular galectin-3 on migration of sarcoma cells on laminin-111. Cell lines from methylcholanthrene-induced sarcomas from both wild type and galectin-3(-/-) mice were established. Despite the presence of similar levels of laminin-binding integrins on the cell surface, galectin-3(-/-) sarcoma cells were more adherent and less migratory than galectin-3(+/+) sarcoma cells on laminin-111. When galectin-3 was transiently expressed in galectin-3(-/-) sarcoma cells, it inhibited cell adhesion and stimulated the migratory response to laminin in a carbohydrate-dependent manner. Extracellular galectin-3 led to the recruitment of SHP-2 phosphatase to focal adhesion plaques, followed by a decrease in the amount of phosphorylated FAK and phospho-paxillin in the lamellipodia of migrating cells. The promigratory activity of extracellular galectin-3 was inhibitable by wortmannin, implicating the activation of a PI-3 kinase dependent pathway in the galectin-3 triggered disruption of adhesion plaques, leading to sarcoma cell migration on laminin-111.
© 2011 Melo et al.
Conflict of interest statement
Figures
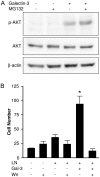
Similar articles
-
Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity.J Biol Chem. 2004 Aug 13;279(33):34922-30. doi: 10.1074/jbc.M312697200. Epub 2004 Jun 17. J Biol Chem. 2004. PMID: 15205467
-
Sustained induction of ERK, protein kinase B, and p70 S6 kinase regulates cell spreading and formation of F-actin microspikes upon ligation of integrins by galectin-8, a mammalian lectin.J Biol Chem. 2003 Apr 18;278(16):14533-42. doi: 10.1074/jbc.M207380200. Epub 2003 Feb 4. J Biol Chem. 2003. Retraction in: J Biol Chem. 2018 May 11;293(19):7265. doi: 10.1074/jbc.W118.003467. PMID: 12569102 Retracted.
-
Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation.Mol Cell Biol. 1997 Aug;17(8):4406-18. doi: 10.1128/MCB.17.8.4406. Mol Cell Biol. 1997. PMID: 9234699 Free PMC article.
-
Role of focal adhesion kinase in integrin signaling.Int J Biochem Cell Biol. 1997 Aug-Sep;29(8-9):1085-96. doi: 10.1016/s1357-2725(97)00051-4. Int J Biochem Cell Biol. 1997. PMID: 9416004 Review.
-
Galectin-3 and the skin.J Dermatol Sci. 2011 Nov;64(2):85-91. doi: 10.1016/j.jdermsci.2011.07.008. Epub 2011 Aug 11. J Dermatol Sci. 2011. PMID: 21889881 Free PMC article. Review.
Cited by
-
Galectin-3 regulation of wound healing and fibrotic processes: insights for chronic skin wound therapeutics.J Cell Commun Signal. 2018 Mar;12(1):281-287. doi: 10.1007/s12079-018-0453-7. Epub 2018 Jan 25. J Cell Commun Signal. 2018. PMID: 29372416 Free PMC article. Review.
-
Calpain Small Subunit Mediated Secretion of Galectin-3 Regulates Traction Stress.Biomedicines. 2024 Jun 4;12(6):1247. doi: 10.3390/biomedicines12061247. Biomedicines. 2024. PMID: 38927454 Free PMC article.
-
Galectin-3: A Positive Regulator of Leukocyte Recruitment in the Inflamed Microcirculation.J Immunol. 2017 Jun 1;198(11):4458-4469. doi: 10.4049/jimmunol.1600709. Epub 2017 Apr 24. J Immunol. 2017. PMID: 28438899 Free PMC article.
-
Galectin-3 Involvement in Cognitive Processes for New Therapeutic Considerations.Front Cell Neurosci. 2022 Jul 5;16:923811. doi: 10.3389/fncel.2022.923811. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35875353 Free PMC article. Review.
-
Extracellular galectin-3 in tumor progression and metastasis.Front Oncol. 2014 Jun 16;4:138. doi: 10.3389/fonc.2014.00138. eCollection 2014. Front Oncol. 2014. PMID: 24982845 Free PMC article. Review.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. - PubMed
-
- Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, et al. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. - PubMed
-
- Jasiulionis MG, Chammas R, Ventura AM, Travassos LR, Brentani RR. alpha6beta1-Integrin, a major cell surface carrier of beta1-6-branched oligosaccharides, mediates migration of EJ-ras-transformed fibroblasts on laminin-1 independently of its glycosylation state. Cancer Res. 1996;56:1682–1689. - PubMed
-
- Wehrle-Haller B, Imhof BA. Integrin-dependent pathologies. J Pathol. 2003;200:481–487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

