Vitamin D3 deficiency differentially affects functional and disease outcomes in the G93A mouse model of amyotrophic lateral sclerosis
- PMID: 22216257
- PMCID: PMC3246470
- DOI: 10.1371/journal.pone.0029354
Vitamin D3 deficiency differentially affects functional and disease outcomes in the G93A mouse model of amyotrophic lateral sclerosis
Abstract
Amyotrophic lateral sclerosis (ALS) is a neuromuscular disease characterized by motor neuron death in the central nervous system. Vitamin D supplementation increases antioxidant activity, reduces inflammation and improves motor neuron survival. We have previously demonstrated that vitamin D(3) supplementation at 10× the adequate intake improves functional outcomes in a mouse model of ALS.
Objective: To determine whether vitamin D deficiency influences functional and disease outcomes in a mouse model of ALS.
Methods: At age 25 d, 102 G93A mice (56 M, 46 F) were divided into two vitamin D(3) groups: 1) adequate (AI; 1 IU D(3)/g feed) and 2) deficient (DEF; 0.025 IU D(3)/g feed). At age 113 d, tibialis anterior (TA), quadriceps (quads) and brain were harvested from 42 mice (22 M and 20 F), whereas the remaining 60 mice (34 M and 26 F) were followed to endpoint.
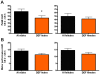
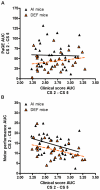
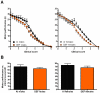
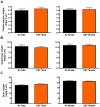
Results: During disease progression, DEF mice had 25% (P=0.022) lower paw grip endurance AUC and 19% (P=0.017) lower motor performance AUC vs. AI mice. Prior to disease onset (CS 2), DEF mice had 36% (P=0.016) lower clinical score (CS) vs. AI mice. DEF mice reached CS 2 six days later vs. AI mice (P=0.004), confirmed by a logrank test which revealed that DEF mice reached CS 2 at a 43% slower rate vs. AI mice (HR= .57; 95% CI: 0.38, 1.74; P=0.002). Body weight-adjusted TA (AI: r=0.662, P=0.001; DEF: r=0.622, P=0.006) and quads (AI: r=0.661, P=0.001; DEF: r=0.768; P<0.001) weights were strongly correlated with age at CS 2.
Conclusion: Vitamin D(3) deficiency improves early disease severity and delays disease onset, but reduces performance in functional outcomes following disease onset, in the high-copy G93A mouse.
© 2011 Solomon et al.
Conflict of interest statement
Figures



Similar articles
-
Dietary Vitamin D3 Restriction Exacerbates Disease Pathophysiology in the Spinal Cord of the G93A Mouse Model of Amyotrophic Lateral Sclerosis.PLoS One. 2015 May 28;10(5):e0126355. doi: 10.1371/journal.pone.0126355. eCollection 2015. PLoS One. 2015. PMID: 26020962 Free PMC article.
-
Vitamin D(3) at 50x AI attenuates the decline in paw grip endurance, but not disease outcomes, in the G93A mouse model of ALS, and is toxic in females.PLoS One. 2013;8(2):e30243. doi: 10.1371/journal.pone.0030243. Epub 2013 Feb 6. PLoS One. 2013. PMID: 23405058 Free PMC article.
-
Dietary vitamin D3 supplementation at 10× the adequate intake improves functional capacity in the G93A transgenic mouse model of ALS, a pilot study.CNS Neurosci Ther. 2012 Jul;18(7):547-57. doi: 10.1111/j.1755-5949.2012.00316.x. Epub 2012 May 17. CNS Neurosci Ther. 2012. PMID: 22591278 Free PMC article.
-
Vitamin D as a potential therapy in amyotrophic lateral sclerosis.CNS Neurosci Ther. 2014 Feb;20(2):101-11. doi: 10.1111/cns.12204. CNS Neurosci Ther. 2014. PMID: 24428861 Free PMC article. Review.
-
Roles of vitamin D in amyotrophic lateral sclerosis: possible genetic and cellular signaling mechanisms.Mol Brain. 2013 Apr 9;6:16. doi: 10.1186/1756-6606-6-16. Mol Brain. 2013. PMID: 23570271 Free PMC article. Review.
Cited by
-
Is There a Role for Vitamin D in Amyotrophic Lateral Sclerosis? A Systematic Review and Meta-Analysis.Front Neurol. 2020 Jul 31;11:697. doi: 10.3389/fneur.2020.00697. eCollection 2020. Front Neurol. 2020. PMID: 32849187 Free PMC article.
-
Complementary and Alternative Therapies in Amyotrophic Lateral Sclerosis.Neurol Clin. 2015 Nov;33(4):909-36. doi: 10.1016/j.ncl.2015.07.008. Epub 2015 Sep 8. Neurol Clin. 2015. PMID: 26515629 Free PMC article. Review.
-
Palliative Care Issues in Amyotrophic Lateral Sclerosis: An Evidenced-Based Review.Am J Hosp Palliat Care. 2016 Feb;33(1):84-92. doi: 10.1177/1049909114548719. Epub 2014 Sep 8. Am J Hosp Palliat Care. 2016. PMID: 25202033 Free PMC article. Review.
-
Guggulsterone Selectively Modulates STAT-3, mTOR, and PPAR-Gamma Signaling in a Methylmercury-Exposed Experimental Neurotoxicity: Evidence from CSF, Blood Plasma, and Brain Samples.Mol Neurobiol. 2024 Aug;61(8):5161-5193. doi: 10.1007/s12035-023-03902-x. Epub 2024 Jan 3. Mol Neurobiol. 2024. PMID: 38170440
-
Vitamin D is Not a Protective Factor in ALS.CNS Neurosci Ther. 2015 Aug;21(8):651-6. doi: 10.1111/cns.12423. Epub 2015 Jun 20. CNS Neurosci Ther. 2015. PMID: 26096806 Free PMC article.
References
-
- Nihei K, McKee AC, Kowall NW. Patterns of neuronal degeneration in the motor cortex of amyotrophic lateral sclerosis patients. Acta Neuropathol. 1993;86:55–64. - PubMed
-
- Zang DW, Cheema SS. Degeneration of corticospinal and bulbospinal systems in the superoxide dismutase 1(G93A) (G1H) transgenic mouse model of familial amyotrophic lateral sclerosis. Neurosci Lett. 2002;332:99–102. - PubMed
-
- deCarvalho M, Matias T, Coelho F, Evangelista T, Pinto A, et al. Motor neuron disease presenting with respiratory failure. J Neurol Sci. 1996;139:117–122. - PubMed
-
- Synofzik M, Fernandez-Santiago R, Maetzler W, Schols L, Andersen PM. The human G93A SOD1 phenotype closely resembles sporadic amyotrophic lateral sclerosis. J Neurol Neurosur Ps. 2010;81:764–767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

