Activity-induced remodeling of olfactory bulb microcircuits revealed by monosynaptic tracing
- PMID: 22216277
- PMCID: PMC3247270
- DOI: 10.1371/journal.pone.0029423
Activity-induced remodeling of olfactory bulb microcircuits revealed by monosynaptic tracing
Abstract
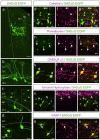
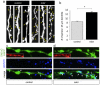
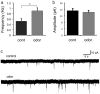
The continued addition of new neurons to mature olfactory circuits represents a remarkable mode of cellular and structural brain plasticity. However, the anatomical configuration of newly established circuits, the types and numbers of neurons that form new synaptic connections, and the effect of sensory experience on synaptic connectivity in the olfactory bulb remain poorly understood. Using in vivo electroporation and monosynaptic tracing, we show that postnatal-born granule cells form synaptic connections with centrifugal inputs and mitral/tufted cells in the mouse olfactory bulb. In addition, newly born granule cells receive extensive input from local inhibitory short axon cells, a poorly understood cell population. The connectivity of short axon cells shows clustered organization, and their synaptic input onto newborn granule cells dramatically and selectively expands with odor stimulation. Our findings suggest that sensory experience promotes the synaptic integration of new neurons into cell type-specific olfactory circuits.
© 2011 Arenkiel et al.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

