Elongase reactions as control points in long-chain polyunsaturated fatty acid synthesis
- PMID: 22216341
- PMCID: PMC3245304
- DOI: 10.1371/journal.pone.0029662
Elongase reactions as control points in long-chain polyunsaturated fatty acid synthesis
Abstract
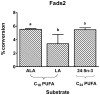
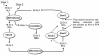
Background: Δ6-Desaturase (Fads2) is widely regarded as rate-limiting in the conversion of dietary α-linolenic acid (18:3n-3; ALA) to the long-chain omega-3 polyunsaturated fatty acid docosahexaenoic acid (22:6n-3; DHA). However, increasing dietary ALA or the direct Fads2 product, stearidonic acid (18:4n-3; SDA), increases tissue levels of eicosapentaenoic acid (20:5n-3; EPA) and docosapentaenoic acid (22:5n-3; DPA), but not DHA. These observations suggest that one or more control points must exist beyond ALA metabolism by Fads2. One possible control point is a second reaction involving Fads2 itself, since this enzyme catalyses desaturation of 24:5n-3 to 24:6n-3, as well as ALA to SDA. However, metabolism of EPA and DPA both require elongation reactions. This study examined the activities of two elongase enzymes as well as the second reaction of Fads2 in order to concentrate on the metabolism of EPA to DHA.
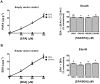
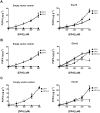
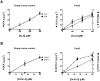
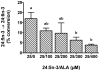
Methodology/principal findings: The substrate selectivities, competitive substrate interactions and dose response curves of the rat elongases, Elovl2 and Elovl5 were determined after expression of the enzymes in yeast. The competitive substrate interactions for rat Fads2 were also examined. Rat Elovl2 was active with C(20) and C(22) polyunsaturated fatty acids and this single enzyme catalysed the sequential elongation reactions of EPA→DPA→24:5n-3. The second reaction DPA→24:5n-3 appeared to be saturated at substrate concentrations not saturating for the first reaction EPA→DPA. ALA dose-dependently inhibited Fads2 conversion of 24:5n-3 to 24:6n-3.
Conclusions: The competition between ALA and 24:5n-3 for Fads2 may explain the decrease in DHA levels observed after certain intakes of dietary ALA have been exceeded. In addition, the apparent saturation of the second Elovl2 reaction, DPA→24:5n-3, provides further explanations for the accumulation of DPA when ALA, SDA or EPA is provided in the diet. This study suggests that Elovl2 will be critical in understanding if DHA synthesis can be increased by dietary means.
© 2011 Gregory et al.
Conflict of interest statement
Figures
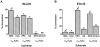
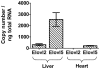
Similar articles
-
Functional characterization of the duck and turkey fatty acyl elongase enzymes ELOVL5 and ELOVL2.J Nutr. 2014 Aug;144(8):1234-9. doi: 10.3945/jn.114.194159. Epub 2014 Jun 11. J Nutr. 2014. PMID: 24919687
-
A complete enzymatic capacity for long-chain polyunsaturated fatty acid biosynthesis is present in the Amazonian teleost tambaqui, Colossoma macropomum.Comp Biochem Physiol B Biochem Mol Biol. 2019 Jan;227:90-97. doi: 10.1016/j.cbpb.2018.09.003. Epub 2018 Oct 2. Comp Biochem Physiol B Biochem Mol Biol. 2019. PMID: 30290221
-
Molecular basis for differential elongation of omega-3 docosapentaenoic acid by the rat Elovl5 and Elovl2.J Lipid Res. 2013 Oct;54(10):2851-7. doi: 10.1194/jlr.M041368. Epub 2013 Jul 21. J Lipid Res. 2013. PMID: 23873268 Free PMC article.
-
Recent advances on the physiological and pathophysiological roles of polyunsaturated fatty acids and their biosynthetic pathway.Biochim Biophys Acta Mol Cell Biol Lipids. 2025 Jan;1870(1):159564. doi: 10.1016/j.bbalip.2024.159564. Epub 2024 Sep 24. Biochim Biophys Acta Mol Cell Biol Lipids. 2025. PMID: 39326727 Review.
-
Desaturase and elongase-limiting endogenous long-chain polyunsaturated fatty acid biosynthesis.Curr Opin Clin Nutr Metab Care. 2016 Mar;19(2):103-10. doi: 10.1097/MCO.0000000000000254. Curr Opin Clin Nutr Metab Care. 2016. PMID: 26828581 Free PMC article. Review.
Cited by
-
How important are fatty acids in human health and can they be used in treating diseases?Gut Microbes. 2024 Jan-Dec;16(1):2420765. doi: 10.1080/19490976.2024.2420765. Epub 2024 Oct 27. Gut Microbes. 2024. PMID: 39462280 Free PMC article. Review.
-
Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA.Front Aging Neurosci. 2015 Apr 21;7:52. doi: 10.3389/fnagi.2015.00052. eCollection 2015. Front Aging Neurosci. 2015. PMID: 25954194 Free PMC article. Review.
-
The gut microbiota promotes hepatic fatty acid desaturation and elongation in mice.Nat Commun. 2018 Sep 14;9(1):3760. doi: 10.1038/s41467-018-05767-4. Nat Commun. 2018. PMID: 30218046 Free PMC article.
-
Fighting Fat With Fat: n-3 Polyunsaturated Fatty Acids and Adipose Deposition in Broiler Chickens.Front Physiol. 2021 Sep 29;12:755317. doi: 10.3389/fphys.2021.755317. eCollection 2021. Front Physiol. 2021. PMID: 34658934 Free PMC article. Review.
-
Unsaturated Fatty Acid Synthesis Is Associated with Worse Survival and Is Differentially Regulated by MYCN and Tumor Suppressor microRNAs in Neuroblastoma.Cancers (Basel). 2024 Apr 21;16(8):1590. doi: 10.3390/cancers16081590. Cancers (Basel). 2024. PMID: 38672672 Free PMC article.
References
-
- Huang YS, Smith RS, Redden PR, Cantrill RC, Horrobin DF. Modification of liver fatty acid metabolism in mice by n-3 and n-6 delta 6-desaturase substrates and products. Biochim Biophys Acta. 1991;1082:319–327. - PubMed
-
- Yamazaki K, Fujikawa M, Hamazaki T, Yano S, Shono T. Comparison of the conversion rates of alpha-linolenic acid (18:3(n - 3)) and stearidonic acid (18:4(n - 3)) to longer polyunsaturated fatty acids in rats. Biochim Biophys Acta. 1992;1123:18–26. - PubMed
-
- James MJ, Ursin VM, Cleland LG. Metabolism of stearidonic acid in human subjects: comparison with the metabolism of other n-3 fatty acids. Am J Clin Nutr. 2003;77:1140–1145. - PubMed
-
- Inagaki K, Aki T, Fukuda Y, Kawamoto S, Shigeta S, et al. Identification and expression of a rat fatty acid elongase involved in the biosynthesis of C18 fatty acids. Biosci Biotechnol Biochem. 2002;66:613–621. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

