Identification of the role of C/EBP in neurite regeneration following microarray analysis of a L. stagnalis CNS injury model
- PMID: 22217148
- PMCID: PMC3315421
- DOI: 10.1186/1471-2202-13-2
Identification of the role of C/EBP in neurite regeneration following microarray analysis of a L. stagnalis CNS injury model
Abstract
Background: Neuronal regeneration in the adult mammalian central nervous system (CNS) is severely compromised due to the presence of extrinsic inhibitory signals and a reduced intrinsic regenerative capacity. In contrast, the CNS of adult Lymnaea stagnalis (L. stagnalis), a freshwater pond snail, is capable of spontaneous regeneration following neuronal injury. Thus, L. stagnalis has served as an animal model to study the cellular mechanisms underlying neuronal regeneration. However, the usage of this model has been limited due to insufficient molecular tools. We have recently conducted a partial neuronal transcriptome sequencing project and reported over 10,000 EST sequences which allowed us to develop and perform a large-scale high throughput microarray analysis.
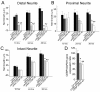
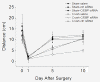
Results: To identify genes that are involved in the robust regenerative capacity observed in L. stagnalis, we designed the first gene chip covering ~15, 000 L. stagnalis CNS EST sequences. We conducted microarray analysis to compare the gene expression profiles of sham-operated (control) and crush-operated (regenerative model) central ganglia of adult L. stagnalis. The expression levels of 348 genes were found to be significantly altered (p < 0.05) following nerve injury. From this pool, 67 sequences showed a greater than 2-fold change: 42 of which were up-regulated and 25 down-regulated. Our qPCR analysis confirmed that CCAAT enhancer binding protein (C/EBP) was up-regulated following nerve injury in a time-dependent manner. In order to test the role of C/EBP in regeneration, C/EBP siRNA was applied following axotomy of cultured Lymnaea PeA neurons. Knockdown of C/EBP following axotomy prevented extension of the distal, proximal and intact neurites. In vivo knockdown of C/EBP postponed recovery of locomotory activity following nerve crush. Taken together, our data suggest both somatic and local effects of C/EBP are involved in neuronal regeneration.
Conclusions: This is the first high-throughput microarray study in L. stagnalis, a model of axonal regeneration following CNS injury. We reported that 348 genes were regulated following central nerve injury in adult L. stagnalis and provided the first evidence for the involvement of local C/EBP in neuronal regeneration. Our study demonstrates the usefulness of the large-scale gene profiling approach in this invertebrate model to study the molecular mechanisms underlying the intrinsic regenerative capacity of adult CNS neurons.
Figures





Similar articles
-
Expression and distribution of transcription factor CCAAT/enhancer-binding protein in the central nervous system of Lymnaea stagnalis.Cell Tissue Res. 2004 Dec;318(3):631-41. doi: 10.1007/s00441-004-0965-8. Epub 2004 Oct 2. Cell Tissue Res. 2004. PMID: 15578275
-
Caltubin, a novel molluscan tubulin-interacting protein, promotes axonal growth and attenuates axonal degeneration of rodent neurons.J Neurosci. 2011 Oct 26;31(43):15231-44. doi: 10.1523/JNEUROSCI.2516-11.2011. J Neurosci. 2011. PMID: 22031869 Free PMC article.
-
Lymnaea epidermal growth factor promotes axonal regeneration in CNS organ culture.J Neurosci. 2001 Dec 1;21(23):9345-54. doi: 10.1523/JNEUROSCI.21-23-09345.2001. J Neurosci. 2001. PMID: 11717368 Free PMC article.
-
Traumatic injury to CNS fiber tracts--what are the genes telling us?Curr Drug Targets. 2004 Oct;5(7):647-54. doi: 10.2174/1389450043345182. Curr Drug Targets. 2004. PMID: 15473254 Review.
-
Targeting neurite growth inhibitors to induce CNS regeneration.Curr Pharm Des. 2005;11(10):1247-53. doi: 10.2174/1381612053507440. Curr Pharm Des. 2005. PMID: 15853681 Review.
Cited by
-
The pond snail Lymnaea stagnalis.Evodevo. 2020 Dec 4;11(1):24. doi: 10.1186/s13227-020-00169-4. Evodevo. 2020. PMID: 33292457 Free PMC article. Review.
-
Tissue-specific evaluation of suitable reference genes for RT-qPCR in the pond snail, Lymnaea stagnalis.PeerJ. 2019 Oct 15;7:e7888. doi: 10.7717/peerj.7888. eCollection 2019. PeerJ. 2019. PMID: 31637135 Free PMC article.
-
Functional Dissection of C. elegans bZip-Protein CEBP-1 Reveals Novel Structural Motifs Required for Axon Regeneration and Nuclear Import.Front Cell Neurosci. 2019 Jul 31;13:348. doi: 10.3389/fncel.2019.00348. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31417366 Free PMC article.
-
Functional characterization of optic photoreception in Lymnaea stagnalis.PLoS One. 2024 Nov 12;19(11):e0313407. doi: 10.1371/journal.pone.0313407. eCollection 2024. PLoS One. 2024. PMID: 39531462 Free PMC article.
-
Transcriptional regulation of the matrix protein Shematrin-2 during shell formation in pearl oyster.J Biol Chem. 2018 Nov 16;293(46):17803-17816. doi: 10.1074/jbc.RA118.005281. Epub 2018 Oct 3. J Biol Chem. 2018. PMID: 30282805 Free PMC article.
References
-
- Aguayo AJ, David S, Bray GM. Influences of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J Exp Biol. 1981;95:231–240. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

