Socioeconomic support reduces nonretention in a comprehensive, community-based antiretroviral therapy program in Uganda
- PMID: 22217680
- PMCID: PMC3887145
- DOI: 10.1097/QAI.0b013e318246e2aa
Socioeconomic support reduces nonretention in a comprehensive, community-based antiretroviral therapy program in Uganda
Abstract
Objectives: We evaluated the benefit of socioeconomic support (S-E support), comprising various financial and nonfinancial services that are available based on assessment of need, in reducing mortality and lost to follow-up (LTFU) at Reach Out Mbuya, a community-based, antiretroviral therapy program in Uganda.
Design: Retrospective observational cohort data from adult patients enrolled between May 31, 2001, and May 31, 2010, were examined.
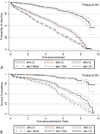
Methods: Patients were categorized into none, 1, and 2 or more S-E support based on the number of different S-E support services they received. Using Cox proportional hazards regression, we modeled the association between S-E support and mortality or LTFU. Kaplan-Meier curves were fitted to examine retention functions stratified by S-E support.
Results: In total, 6654 patients were evaluated. After 10 years, 2700 (41%) were retained. Of the 3954 not retained, 2933 (74%) were LTFU and 1021 (26%) had died. After 1, 2, 5, and 10 years, the risks of LTFU or mortality in patients who received no S-E support were significantly higher than those who received some S-E support. In adjusted hazards ratios, patients who received no S-E support were 1.5-fold (1.39-1.64) and 6.7-fold (5.56-7.69) more likely to get LTFU compared with those who received 1 or ≥ 2 S-E support, respectively. Likewise, patients who received no S-E support were 1.5-fold (confidence interval: 1.16 to 1.89) and 4.3-fold (confidence interval: 2.94 to 6.25) more likely to die compared with those who received 1 or 2+ S-E support, respectively.
Conclusions: Provision of S-E support reduced LTFU and mortality, suggesting the value of incorporating such strategies for promoting continuity of care.
Conflict of interest statement
The authors have no funding or conflicts of interest to disclose.
Figures
Similar articles
-
Outcomes of Patients Lost to Follow-up in African Antiretroviral Therapy Programs: Individual Patient Data Meta-analysis.Clin Infect Dis. 2018 Nov 13;67(11):1643-1652. doi: 10.1093/cid/ciy347. Clin Infect Dis. 2018. PMID: 29889240 Free PMC article.
-
Retention of HIV infected pregnant and breastfeeding women on option B+ in Gomba District, Uganda: a retrospective cohort study.BMC Infect Dis. 2018 Oct 24;18(1):533. doi: 10.1186/s12879-018-3450-9. BMC Infect Dis. 2018. PMID: 30355356 Free PMC article.
-
Determinants of loss to follow-up among HIV positive patients receiving antiretroviral therapy in a test and treat setting: A retrospective cohort study in Masaka, Uganda.PLoS One. 2020 Apr 7;15(4):e0217606. doi: 10.1371/journal.pone.0217606. eCollection 2020. PLoS One. 2020. PMID: 32255796 Free PMC article.
-
Return to normal life after AIDS as a reason for lost to follow-up in a community-based antiretroviral treatment program.J Acquir Immune Defic Syndr. 2012 Jun 1;60(2):e36-45. doi: 10.1097/FTD.0b013e3182526e6a. J Acquir Immune Defic Syndr. 2012. PMID: 22622076 Free PMC article.
-
High rates of loss to follow-up during the first year of pre-antiretroviral therapy for HIV patients at sites providing pre-ART care in Nigeria, 2004-2012.PLoS One. 2017 Sep 1;12(9):e0183823. doi: 10.1371/journal.pone.0183823. eCollection 2017. PLoS One. 2017. PMID: 28863160 Free PMC article.
Cited by
-
Outcomes of HIV-positive patients lost to follow-up in African treatment programmes.Trop Med Int Health. 2017 Apr;22(4):375-387. doi: 10.1111/tmi.12843. Epub 2017 Feb 20. Trop Med Int Health. 2017. PMID: 28102610 Free PMC article.
-
HIV care policy in India: A review of social security schemes.J Family Med Prim Care. 2022 May;11(5):1648-1657. doi: 10.4103/jfmpc.jfmpc_1755_21. Epub 2022 May 14. J Family Med Prim Care. 2022. PMID: 35800572 Free PMC article. Review.
-
Creating cohesive communities: using Conditional-Collective-Community-Based Incentives to change social norms on polio immunization in Pakistan.Front Public Health. 2025 Jun 3;13:1575319. doi: 10.3389/fpubh.2025.1575319. eCollection 2025. Front Public Health. 2025. PMID: 40529690 Free PMC article.
-
Outcomes of Patients Lost to Follow-up in African Antiretroviral Therapy Programs: Individual Patient Data Meta-analysis.Clin Infect Dis. 2018 Nov 13;67(11):1643-1652. doi: 10.1093/cid/ciy347. Clin Infect Dis. 2018. PMID: 29889240 Free PMC article.
-
Explaining antiretroviral therapy adherence success among HIV-infected children in rural Uganda: a qualitative study.AIDS Behav. 2015 Apr;19(4):584-93. doi: 10.1007/s10461-014-0924-7. AIDS Behav. 2015. PMID: 25323679 Free PMC article.
References
-
- Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. - PubMed
-
- Mocroft A, Vella S, Benfield TL, et al. Changing patterns of mortality across Europe in patients infected with HIV-1. EuroSIDA Study Group. Lancet. 1998;352:1725–1730. - PubMed
-
- Sterne JA, Hernan MA, Ledergerber B, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet. 2005;366:378–384. - PubMed
-
- Weidle PJ, Wamai N, Solberg P, et al. Adherence to antiretroviral therapy in a home-based AIDS care programme in rural Uganda. Lancet. 2006;368:1587–1594. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

