Regulation of AMPA receptor trafficking and synaptic plasticity
- PMID: 22217700
- PMCID: PMC3392447
- DOI: 10.1016/j.conb.2011.12.006
Regulation of AMPA receptor trafficking and synaptic plasticity
Abstract
AMPA receptors (AMPARs) mediate the majority of fast excitatory synaptic transmission in the brain. Dynamic changes in neuronal synaptic efficacy, termed synaptic plasticity, are thought to underlie information coding and storage in learning and memory. One major mechanism that regulates synaptic strength involves the tightly regulated trafficking of AMPARs into and out of synapses. The life cycle of AMPARs from their biosynthesis, membrane trafficking, and synaptic targeting to their degradation are controlled by a series of orchestrated interactions with numerous intracellular regulatory proteins. Here we review recent progress made toward the understanding the regulation of AMPAR trafficking, focusing on the roles of several key intracellular AMPAR interacting proteins.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
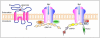

References
-
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. - PubMed
-
-
Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. * This study provides a molecular quantification of both synaptic and extrasynaptic subunit composition of AMPARs in hippocampal CA1 pyramidal neurons.
-
-
- Correia SS, Bassani S, Brown TC, Lise MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat Neurosci. 2008;11:457–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

