N-Amino acid linoleoyl conjugates: anti-inflammatory activities
- PMID: 22217875
- PMCID: PMC3258367
- DOI: 10.1016/j.bmcl.2011.12.040
N-Amino acid linoleoyl conjugates: anti-inflammatory activities
Abstract
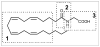
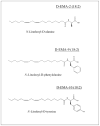
Several N-linked amino acid-linoleic acid conjugates were studied for their potential as anti inflammatory agents. The parent molecule, N-linoleoylglycine was tested in an in vivo model, the mouse peritonitis assay where it showed activity in reducing leukocyte migration at doses as low as 0.3mg/kg when administered by mouth in safflower oil. Harvested peritoneal cells produced elevated levels of the inflammation-resolving eicosanoid 15-deoxy-Δ(13,14)-PGJ(2). These results are similar to those obtained in earlier studies with N-arachidonoylglycine. An in vitro model using mouse macrophage RAW cells was used to evaluate a small group of structural analogs for their ability to stimulate 15-deoxy-Δ(13,14)-PGJ(2) production. The d-alanine derivative was the most active while the d-phenylalanine showed almost no response. A high degree of stereo specificity was observed comparing the d and l alanine isomers; the latter being the less active. It was concluded that linoleic acid conjugates could provide suitable templates in a drug discovery program leading to novel agents for promoting the resolution of chronic inflammation.
Copyright © 2011. Published by Elsevier Ltd.
Figures



Similar articles
-
Single Amino Acid Substitutions at Specific Positions of the Heptad Repeat Sequence of Piscidin-1 Yielded Novel Analogs That Show Low Cytotoxicity and In Vitro and In Vivo Antiendotoxin Activity.Antimicrob Agents Chemother. 2016 May 23;60(6):3687-99. doi: 10.1128/AAC.02341-15. Print 2016 Jun. Antimicrob Agents Chemother. 2016. PMID: 27067326 Free PMC article.
-
LL-37-derived short antimicrobial peptide KR-12-a5 and its d-amino acid substituted analogs with cell selectivity, anti-biofilm activity, synergistic effect with conventional antibiotics, and anti-inflammatory activity.Eur J Med Chem. 2017 Aug 18;136:428-441. doi: 10.1016/j.ejmech.2017.05.028. Epub 2017 May 11. Eur J Med Chem. 2017. PMID: 28525841
-
β-Amino acid and amino-alcohol conjugation of a nonsteroidal anti-inflammatory drug (NSAID) imparts hydrogelation displaying remarkable biostability, biocompatibility, and anti-inflammatory properties.Langmuir. 2013 Aug 13;29(32):10254-63. doi: 10.1021/la401929v. Epub 2013 Aug 1. Langmuir. 2013. PMID: 23859562
-
Synthesis and anti-phlogistic potency of some new non-proteinogenic amino acid conjugates of "Diclofenac".Amino Acids. 1999;16(3-4):425-40. doi: 10.1007/BF01388181. Amino Acids. 1999. PMID: 10399025 Review.
-
Novel anti-inflammatory--pro-resolving mediators and their receptors.Curr Top Med Chem. 2011;11(6):629-47. doi: 10.2174/1568026611109060629. Curr Top Med Chem. 2011. PMID: 21261595 Free PMC article. Review.
Cited by
-
Navy Bean and Rice Bran Intake Alters the Plasma Metabolome of Children at Risk for Cardiovascular Disease.Front Nutr. 2018 Jan 19;4:71. doi: 10.3389/fnut.2017.00071. eCollection 2017. Front Nutr. 2018. PMID: 29404331 Free PMC article.
-
Asymmetric synthesis of novel N-(1-phenyl-2,3-dihydroxypropyl)arachidonylamides and evaluation of their anti-inflammatory activity.Life Sci. 2013 Mar 19;92(8-9):506-11. doi: 10.1016/j.lfs.2012.06.040. Epub 2012 Jul 20. Life Sci. 2013. PMID: 22820546 Free PMC article.
-
The Biology of Veganism: Plasma Metabolomics Analysis Reveals Distinct Profiles of Vegans and Non-Vegetarians in the Adventist Health Study-2 Cohort.Nutrients. 2022 Feb 8;14(3):709. doi: 10.3390/nu14030709. Nutrients. 2022. PMID: 35277064 Free PMC article.
-
N-FATTY ACYLGLYCINES: UNDERAPPRECIATED ENDOCANNABINOID-LIKE FATTY ACID AMIDES?J Biol Nat. 2017;8(4):156-165. Epub 2018 Mar 3. J Biol Nat. 2017. PMID: 29607420 Free PMC article.
-
New Inducible Nitric Oxide Synthase and Cyclooxygenase-2 Inhibitors, Nalidixic Acid Linked to Isatin Schiff Bases via Certain l-Amino Acid Bridges.Molecules. 2016 Apr 15;21(4):498. doi: 10.3390/molecules21040498. Molecules. 2016. PMID: 27092477 Free PMC article.
References
-
- Huang SM, Bisogno T, Petros TJ, Chang SY, Zavitsanos PA, Zipkin RE, Sivakumar R, Coop A, Maeda DY, De Petrocellis L, Burstein S, Di Marzo V, Walker JM. J Biol Chem. 2001;276:42639. - PubMed
-
- Burstein SMA, Pearson W, Rooney T, Yagen B, Zipkin R, Zurier A. Studies with analogs of anandamide and indomethacin. International Cannabinoid Research Society; Burlington, VT: 1997.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

