Increased Kv1 channel expression may contribute to decreased sIPSC frequency following chronic inhibition of NR2B-containing NMDAR
- PMID: 22218089
- PMCID: PMC3327840
- DOI: 10.1038/npp.2011.320
Increased Kv1 channel expression may contribute to decreased sIPSC frequency following chronic inhibition of NR2B-containing NMDAR
Abstract
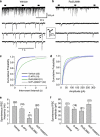
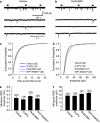
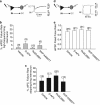
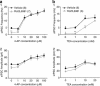
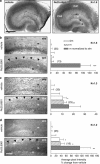
Numerous studies have documented the effects of chronic N-methyl-D-aspartate receptor (NMDAR) blockade on excitatory circuits, but the effects on inhibitory circuitry are not well studied. NR2A- and NR2B-containing NMDARs play differential roles in physiological processes, but the consequences of chronic NR2A- or NR2B-containing NMDAR inhibition on glutamatergic and GABAergic neurotransmission are unknown. We investigated altered GABAergic neurotransmission in dentate granule cells and interneurons following chronic treatment with the NR2B-selective antagonist, Ro25,6981, the NR2A-prefering antagonist, NVP-AAM077, or the non-subunit-selective NMDAR antagonist, D-APV, in organotypic hippocampal slice cultures. Electrophysiological recordings revealed large reductions in spontaneous inhibitory postsynaptic current (sIPSC) frequency in both granule cells and interneurons following chronic Ro25,6981 treatment, which was associated with minimally altered sIPSC amplitude, miniature inhibitory postsynaptic current (mIPSC) frequency, and mIPSC amplitude, suggesting diminished action potential-dependent GABA release. Chronic NVP-AAM077 or D-APV treatment had little effect on these measures. Reduced sIPSC frequency did not arise from downregulated GABA(A)R, altered excitatory or inhibitory drive to interneurons, altered interneuron membrane properties, increased failure rate, decreased action potential-dependent release probability, or mGluR/GABA(B) receptor modulation of GABA release. However, chronic Ro25,6981-mediated reductions in sIPSC frequency were occluded by the K+ channel blockers, dendrotoxin, margatoxin, and agitoxin, but not dendrotoxin-K or XE991. Immunohistochemistry also showed increased Kv1.2, Kv1.3, and Kv1.6 in the dentate molecular layer following chronic Ro25,6981 treatment. Our findings suggest that increased Kv1 channel expression/function contributed to diminished action potential-dependent GABA release following chronic NR2B-containing NMDAR inhibition and that these Kv1 channels may be heteromeric complexes containing Kv1.2, Kv1.3, and Kv1.6.
Figures





References
-
- Akhtar S, Shamotienko O, Papakosta M, Ali F, Dolly JO. Characteristics of brain Kv1 channels tailored to mimic native counterparts by tandem linkage of alpha subunits: implications for K+ channelopathies. J Biol Chem. 2002;277:16376–16382. - PubMed
-
- Arnold DB. Polarized targeting of ion channels in neurons. Pflugers Arch. 2007;453:763–769. - PubMed
-
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett. 2002;12:1099–1102. - PubMed
-
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

