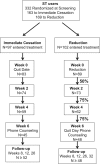
Comparing an immediate cessation versus reduction approach to smokeless tobacco cessation
- PMID: 22218402
- PMCID: PMC3530899
- DOI: 10.1093/ntr/ntr302
Comparing an immediate cessation versus reduction approach to smokeless tobacco cessation
Abstract
Introduction: Relatively few studies have investigated pharmacological or behavioral treatment of smokeless tobacco (ST) users who do not have immediate quit plans. In this study, we compared a reduction treatment approach with an immediate cessation approach in a population of ST users who reported no immediate plans to quit.
Methods: Subjects randomly assigned to the immediate cessation condition set a quit date soon after enrollment and were offered 2 weeks of nicotine patch therapy to help in their cessation efforts. Subjects assigned to the ST reduction group were provided with their choice of either 4 mg nicotine lozenge or ST brand switching to help them reduce their ST use or levels of nicotine exposure, respectively. Quit date was 6 weeks after the onset of treatment. Follow-up was at 12 weeks and 26 weeks postenrollment and 26 weeks postquit.
Results: Both 7-day point prevalence abstinence and prolonged abstinence rates following the quit date were significantly higher in the immediate cessation group versus the reduction group at 12 and 26 weeks (all p values ≤ .04) and for prolonged abstinence at 6 months postquit (p = .002). Significant reductions in ST use among nonquitters were observed for both groups (p < .0001) with no differences between groups.
Conclusion: Our study demonstrated that immediate cessation with an established quit date resulted in greater cessation success than a gradual reduction approach among ST users who do not have an immediate quit plan but are motivated to quit.
Figures
References
-
- Bile KMLO, Shaikh JA, Afridi HU, Khan Y. Smokeless tobacco use in Pakistan and its association with oropharyngeal cancer. Eastern Mediterranean Health Journal. 2010;16(Suppl.):S24–S30. - PubMed
-
- Boffetta P, Hecht S, Gray N, Gupta P, Straif K. Smokeless tobacco and cancer. Lancet Oncology. 2008;9:667–675. doi:10.1016/S1470-2045(08)70173-6. - PubMed
-
- Boyle RG, Enstad C, Asche SE, Thoele MJ, Sherwood NE, Severson HH, et al. A randomized controlled trial of Telephone Counseling with smokeless tobacco users: The ChewFree Minnesota study. Nicotine & Tobacco Research. 2008;10:1433–1440. doi:10.1080/14622200802279872. - PubMed
-
- Boyle RG, Pronk NP, Enstad CJ. A randomized trial of telephone counseling with adult moist snuff users. American Journal of Health Behavior. 2004;28:347–351. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical