Chronic hyponatremia exacerbates multiple manifestations of senescence in male rats
- PMID: 22218780
- PMCID: PMC3592950
- DOI: 10.1007/s11357-011-9347-9
Chronic hyponatremia exacerbates multiple manifestations of senescence in male rats
Abstract
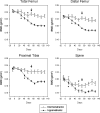
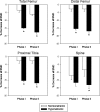
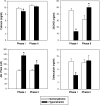
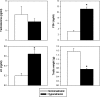
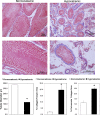
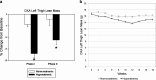
The syndrome of inappropriate antidiuretic hormone secretion (SIADH) is frequently responsible for chronic hyponatremia in the elderly due to age-related disruption of the inhibitory component of brain osmoregulatory mechanisms. Recent research has indicated that chronic hyponatremia is associated with gait disturbances, increased falls, and bone fragility in humans, and we have found that chronic hyponatremia causes increased bone resorption and reduced bone mineral density in young rats. In this study, we used a model of SIADH to study multi-organ consequences of chronic hyponatremia in aged rats. Sustained hyponatremia for 18 weeks caused progressive reduction of bone mineral density by DXA and decreased bone ash calcium, phosphate and sodium contents at the tibia and lumbar vertebrae. Administration of 10-fold higher vitamin D during the last 8 weeks of the study compensated for the reduction in bone formation and halted bone loss. Hyponatremic rats developed hypogonadism, as indicated by slightly lower serum testosterone and higher serum FSH and LH concentrations, markedly decreased testicular weight, and abnormal testicular histology. Aged hyponatremic rats also manifested decreased body fat, skeletal muscle sarcopenia by densitometry, and cardiomyopathy manifested as increased heart weight and perivascular and interstitial fibrosis by histology. These findings are consistent with recent results in cultured osteoclastic cells, indicating that low extracellular sodium concentrations increased oxidative stress, thereby potentially exacerbating multiple manifestations of senescence. Future prospective studies in patients with SIADH may indicate whether these multi-organ age-related comorbidities may potentially contribute to the observed increased incidence of fractures and mortality in this population.
Figures

References
-
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution by age-associated oxidative stress and its acceleration by loss of sex steroids. J Biol Chem. 2007;282:27285–27297. doi: 10.1074/jbc.M702810200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
