Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus
- PMID: 22219028
- PMCID: PMC3331663
- DOI: 10.1152/jn.00715.2011
Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus
Abstract
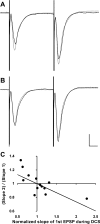
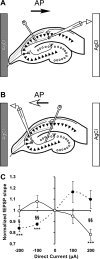
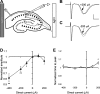
Transcranial direct current stimulation (tDCS) is a method for modulating cortical excitability by weak constant electrical current that is applied through scalp electrodes. Although often described in terms of anodal or cathodal stimulation, depending on which scalp electrode pole is proximal to the cortical region of interest, it is the orientation of neuronal structures relative to the direct current (DC) vector that determines the effect of tDCS. To investigate the contribution of neural pathway orientation, we studied DCS-mediated neuromodulation in an in vitro rat hippocampal slice preparation. We examined the contribution of dendritic orientation to the direct current stimulation (DCS) neuromodulatory effect by recording field excitatory postsynaptic potentials (fEPSPs) in apical and basal dendrites of CA1 neurons within a constant DC field. In addition, we assessed the contribution of axonal orientation by recording CA1 and CA3 apical fEPSPs generated by stimulation of oppositely oriented Schaffer collateral and mossy fiber axons, respectively, during DCS. Finally, nonsynaptic excitatory signal propagation was measured along antidromically stimulated CA1 axons at different DCS amplitudes and polarity. We find that modulation of both the fEPSP and population spike depends on axonal orientation relative to the electric field vector. Axonal orientation determines whether the DC field is excitatory or inhibitory and dendritic orientation affects the magnitude, but not the overall direction, of the DC effect. These data suggest that tDCS may oppositely affect neurons in a stimulated cortical volume if these neurons are excited by oppositely orientated axons in a constant electrical field.
Figures





References
-
- Antal A, Kriener N, Lang N, Boros K, Paulus W. Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia 31: 820–828, 2011 - PubMed
-
- Brunoni AR, Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Boggio PS, Giacopuzzi M, Barbieri S, Priori A. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 35: 96–101 - PubMed
-
- Buckmaster PS, Wong EH. Evoked responses of the dentate gyrus during seizures in developing gerbils with inherited epilepsy. J Neurophysiol 88: 783–793, 2002 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

