Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions
- PMID: 22219194
- PMCID: PMC3307260
- DOI: 10.1074/jbc.M111.337345
Myeloperoxidase targets apolipoprotein A-I, the major high density lipoprotein protein, for site-specific oxidation in human atherosclerotic lesions
Abstract
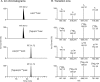
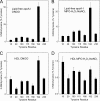
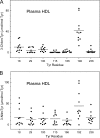
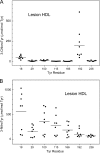
Oxidative damage by myeloperoxidase (MPO) has been proposed to deprive HDL of its cardioprotective effects. In vitro studies reveal that MPO chlorinates and nitrates specific tyrosine residues of apoA-I, the major HDL protein. After Tyr-192 is chlorinated, apoA-I is less able to promote cholesterol efflux by the ABCA1 pathway. To investigate the potential role of this pathway in vivo, we used tandem mass spectrometry with selected reaction monitoring to quantify the regiospecific oxidation of apoA-I. This approach demonstrated that Tyr-192 is the major chlorination site in apoA-I in both plasma and lesion HDL of humans. We also found that Tyr-192 is the major nitration site in apoA-I of circulating HDL but that Tyr-18 is the major site in lesion HDL. Levels of 3-nitrotyrosine strongly correlated with levels of 3-chlorotyrosine in lesion HDL, and Tyr-18 of apoA-I was the major nitration site in HDL exposed to MPO in vitro, suggesting that MPO is the major pathway for chlorination and nitration of HDL in human atherosclerotic tissue. These observations may have implications for treating cardiovascular disease, because recombinant apoA-I is under investigation as a therapeutic agent and mutant forms of apoA-I that resist oxidation might be more cardioprotective than the native form.
Figures
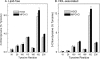


References
-
- Gordon D. J., Rifkind B. M. (1989) High density lipoprotein. The clinical implications of recent studies. N. Engl. J. Med. 321, 1311–1316 - PubMed
-
- Movva R., Rader D. J. (2008) Laboratory assessment of HDL heterogeneity and function. Clin Chem. 54, 788–800 - PubMed
-
- Oram J. F., Heinecke J. W. (2005) ATP binding cassette transporter A1. A cell cholesterol exporter that protects against cardiovascular disease. Physiol. Rev. 85, 1343–1372 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK089503/DK/NIDDK NIH HHS/United States
- R01 HL085437/HL/NHLBI NIH HHS/United States
- R01 HL077268/HL/NHLBI NIH HHS/United States
- R01HL085437/HL/NHLBI NIH HHS/United States
- P30 DK089503/DK/NIDDK NIH HHS/United States
- R01 HL086798/HL/NHLBI NIH HHS/United States
- P30DK017047/DK/NIDDK NIH HHS/United States
- P30 DK020572/DK/NIDDK NIH HHS/United States
- R00 HL091055/HL/NHLBI NIH HHS/United States
- R01HL086798/HL/NHLBI NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- R00HL091055/HL/NHLBI NIH HHS/United States
- R01HL077268/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

