Tumor necrosis factor (TNF) receptor-associated factor 7 is required for TNFα-induced Jun NH2-terminal kinase activation and promotes cell death by regulating polyubiquitination and lysosomal degradation of c-FLIP protein
- PMID: 22219201
- PMCID: PMC3285372
- DOI: 10.1074/jbc.M111.300137
Tumor necrosis factor (TNF) receptor-associated factor 7 is required for TNFα-induced Jun NH2-terminal kinase activation and promotes cell death by regulating polyubiquitination and lysosomal degradation of c-FLIP protein
Abstract
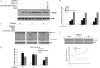
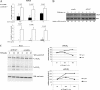
The pro-inflammatory cytokine tumor necrosis factor (TNF) α signals both cell survival and death. The biological outcome of TNFα treatment is determined by the balance between survival factors and Jun NH(2)-terminal kinase (JNK) signaling, which promotes cell death. Here, we show that TRAF7, the most recently identified member of the TNF receptor-associated factors (TRAFs) family of proteins, is essential for activation of JNK following TNFα stimulation. We also show that TRAF6 and TRAF7 promote unconventional polyubiquitination of the anti-apoptotic protein c-FLIP(L) and demonstrate that degradation of c-FLIP(L) also occurs through a lysosomal pathway. RNA interference-mediated depletion of TRAF7 correlates with increased c-FLIP(L) expression level, which, in turn, results in resistance to TNFα cytotoxicity. Collectively, our results indicate an important role for TRAF7 in the activation of JNK following TNFα stimulation and clearly point to an involvement of this protein in regulating the turnover of c-FLIP and, consequently, cell death.
Figures



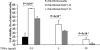
References
-
- Wajant H., Pfizenmaier K., Scheurich P. (2003) Tumor necrosis factor signaling. Cell Death Differ. 10, 45–65 - PubMed
-
- Peter M. E., Krammer P. H. (2003) The CD95 (APO-1/Fas) DISC and beyond. Cell Death Differ. 10, 26–35 - PubMed
-
- Boatright K. M., Renatus M., Scott F. L., Sperandio S., Shin H., Pedersen I. M., Ricci J. E., Edris W. A., Sutherlin D. P., Green D. R., Salvesen G. S. (2003) A unified model for apical caspase activation. Mol. Cell 11, 529–541 - PubMed
-
- Donepudi M., Mac Sweeney A., Briand C., Grütter M. G. (2003) Insights into the regulatory mechanism for caspase-8 activation. Mol. Cell 11, 543–549 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

