The autoimmune regulator prevents premature reproductive senescence in female mice
- PMID: 22219212
- PMCID: PMC3338656
- DOI: 10.1095/biolreprod.111.097501
The autoimmune regulator prevents premature reproductive senescence in female mice
Abstract
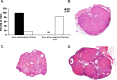
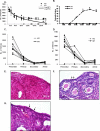
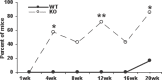
Loss-of-function mutations in the autoimmune regulator (AIRE) gene are responsible for autoimmune polyglandular syndrome type 1 (APS-1), which commonly manifests as infertility in women. AIRE is a transcriptional regulator that promotes expression of tissue-restricted antigens in the thymus, including antigens specific to the ovary. Thymic expression of ovarian genes under AIRE's control may be critical for preventing ovarian autoimmune disease. Because mice lacking Aire are an important APS-1 model, we examined the reproductive properties of female Aire-deficient (Aire(-/-)) mice. Female Aire(-/-) mice on the BALB/c background were examined for reproductive parameters, including fertility, litter sizes, and ovarian follicular reserves. Although delayed puberty was observed in Aire(-/-) mice, all mice entered puberty and exhibited mating behavior. Only 50% of Aire(-/-) females gave an initial litter, and only 16% were able to produce two litters. Ovarian histopathologic examination revealed that 83% of previously bred females lost all ovarian follicular reserves. Among virgin females, follicular depletion was observed in 25% by 8 wk, and by 20 wk, 50%-60% of mice lost all follicles. This was associated with elevated serum follicle-stimulating hormone level and ovarian infiltration of proliferating CD3+ T lymphocytes. Ovulation rates of 6-wk-old Aire(-/-) mice were reduced by 22%, but this difference was not statistically significant. Finally, transplantation experiments revealed that follicular loss depended on factors extrinsic to the ovary. These results suggest that immune-mediated ovarian follicular depletion is a mechanism of infertility in Aire(-/-) mice. The results have important implications in the pathogenesis of ovarian autoimmune disease in women.
Figures
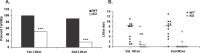

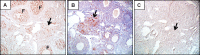


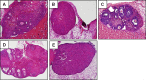
Comment in
-
Evidence that the autoimmune regulator gene influences thymic production of ovarian antigens and prevents autoimmune-mediated premature reproductive senescence.Biol Reprod. 2012 Apr 12;86(4):109. doi: 10.1095/biolreprod.112.099242. Print 2012 Apr. Biol Reprod. 2012. PMID: 22278984 No abstract available.
References
-
- Forges T, Monnier-Barbarino P, Faure GC, Bene MC. Autoimmunity and antigenic targets in ovarian pathology. Hum Reprod Update 2004; 10: 163 175 - PubMed
-
- Conway GS, Kaltsas G, Patel A, Davies MC, Jacobs HS. Characterization of idiopathic premature ovarian failure. Fertil Steril 1996; 65: 337 341 - PubMed
-
- Kyewski B, Klein L. A central role for central tolerance. Annu Rev Immunol 2006; 24: 571 606 - PubMed
-
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol 2003; 21: 139 176 - PubMed
-
- Smith KM, Olson DC, Hirose R, Hanahan D. Pancreatic gene expression in rare cells of thymic medulla: evidence for functional contribution to T cell tolerance. Int Immunol 1997; 9: 1355 1365 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

