EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation
- PMID: 22219286
- PMCID: PMC6621327
- DOI: 10.1523/JNEUROSCI.4792-11.2012
EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation
Abstract
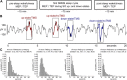
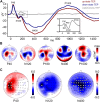
Evoked cortical responses do not follow a rigid input-output function but are dynamically shaped by intrinsic neural properties at the time of stimulation. Recent research has emphasized the role of oscillatory activity in determining cortical excitability. Here we employed EEG-guided transcranial magnetic stimulation (TMS) during non-rapid eye movement sleep to examine whether the spontaneous <1 Hz neocortical slow oscillation (SO) is associated with corresponding fluctuations of evoked responses. Whereas the SO's alternating phases of global depolarization (up-state) and hyperpolarization (down-state) are clearly associated with fluctuations in spontaneous neuronal excitation, less is known about state-dependent shifts in neocortical excitability. In 12 human volunteers, single-pulse TMS of the primary motor cortical hand area (M1(HAND)) was triggered online by automatic detection of SO up-states and down-states in the EEG. State-dependent changes in cortical excitability were traced by simultaneously recording motor-evoked potentials (MEPs) and TMS-evoked EEG potentials (TEPs). Compared to wakefulness and regardless of SO state, sleep MEPs were smaller and delayed whereas sleep TEPs were fundamentally altered, closely resembling a spontaneous SO. However, both MEPs and TEPs were consistently larger when evoked during SO up-states than during down-states, and amplitudes within each SO state depended on the actual EEG potential at the time and site of stimulation. These results provide first-time evidence for a rapid state-dependent shift in neocortical excitability during a neuronal oscillation in the human brain. We further demonstrate that EEG-guided temporal neuronavigation is a powerful tool to investigate the phase-dependent effects of neuronal oscillations on perception, cognition, and motor control.
Figures





References
-
- Achermann P, Borbély AA. Low-frequency (<1 Hz) oscillations in the human sleep electroencephalogram. Neuroscience. 1997;81:213–222. - PubMed
-
- Avesani M, Formaggio E, Fuggetta G, Fiaschi A, Manganotti P. Corticospinal excitability in human subjects during nonrapid eye movement sleep: single and paired-pulse transcranial magnetic stimulation study. Exp Brain Res. 2008;187:17–23. - PubMed
-
- Bergmann TO, Mölle M, Marshall L, Kaya-Yildiz L, Born J, Siebner RH. A local signature of LTP- and LTD-like plasticity in human NREM sleep. Eur J Neurosci. 2008;27:2241–2249. - PubMed
-
- Bertini M, Ferrara M, De Gennaro L, Curcio G, Fratello F, Romei V, Pauri F, Rossini PM. Corticospinal excitability and sleep: a motor threshold assessment by transcranial magnetic stimulation after awakenings from REM and NREM sleep. J Sleep Res. 2004;13:31–36. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
