A reduced-dose seasonal trivalent influenza vaccine is safe and immunogenic in adult and elderly patients in a randomized controlled trial
- PMID: 22219315
- PMCID: PMC3294605
- DOI: 10.1128/CVI.05619-11
A reduced-dose seasonal trivalent influenza vaccine is safe and immunogenic in adult and elderly patients in a randomized controlled trial
Abstract
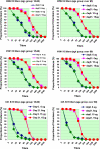
With the recent pandemic of influenza A (H1N1) and vaccine shortages, there has been considerable interest in developing influenza vaccines with reduced doses, allowing for increased production capacity. Here we report a prospective, randomized, double-blind, single-center clinical trial of a reduced-dose whole-virion inactivated, adjuvanted influenza vaccine in adult and elderly volunteers. A total of 234 subjects, including 120 adults (18 to 60 years of age) and 114 elderly subjects (>60 years of age) were enrolled to receive either 6 μg or the conventional 15-μg dose of seasonal trivalent influenza vaccines. The subjects were followed for safety analysis, and serum samples were obtained to assess immunogenicity by hemagglutination inhibition testing. The subjects developed antibody responses against the seasonal influenza A virus H1N1 and H3N2 strains, as well as the seasonal influenza B virus included in the vaccines. Single doses of 6 μg fulfilled licensing criteria for seasonal influenza vaccines. No significant differences in rates of seroconversion or seroprotection or in geometric mean titers were found between the two dosage levels. All adverse events were rare, mild, and transient. We found that the present reduced-dose vaccine is safe and immunogenic in healthy adult and elderly subjects and triggers immune responses that comply with licensing criteria.
Figures
Similar articles
-
Immunogenicity and safety of an inactivated 2012/2013 trivalent influenza vaccine produced in mammalian cell culture (Optaflu®): an open label, uncontrolled study.Hum Vaccin Immunother. 2014;10(2):441-8. doi: 10.4161/hv.27140. Epub 2013 Nov 15. Hum Vaccin Immunother. 2014. PMID: 24240428 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a seasonal trivalent inactivated split influenza vaccine: a phase I randomized clinical trial in healthy Serbian adults.Hum Vaccin Immunother. 2018 Mar 4;14(3):579-586. doi: 10.1080/21645515.2017.1415683. Epub 2018 Feb 23. Hum Vaccin Immunother. 2018. PMID: 29239682 Free PMC article. Clinical Trial.
-
Safety, immunogenicity and cross-reactivity of a Northern hemisphere 2013-2014 seasonal trivalent inactivated split influenza virus vaccine, Anflu®.Hum Vaccin Immunother. 2016 May 3;12(5):1229-34. doi: 10.1080/21645515.2015.1123357. Epub 2016 Mar 2. Hum Vaccin Immunother. 2016. PMID: 26934750 Free PMC article. Clinical Trial.
-
Inactivated split-virion seasonal influenza vaccine (Fluarix): a review of its use in the prevention of seasonal influenza in adults and the elderly.Drugs. 2010 Aug 20;70(12):1519-43. doi: 10.2165/11205020-000000000-00000. Drugs. 2010. PMID: 20687619 Review.
-
Review of 10 years of clinical experience with Chinese domestic trivalent influenza vaccine Anflu®.Hum Vaccin Immunother. 2014;10(1):73-82. doi: 10.4161/hv.26715. Epub 2013 Oct 8. Hum Vaccin Immunother. 2014. PMID: 24104060 Free PMC article. Review.
Cited by
-
Acute exercise enhancement of pneumococcal vaccination response: a randomised controlled trial of weaker and stronger immune response.Vaccine. 2012 Oct 5;30(45):6389-95. doi: 10.1016/j.vaccine.2012.08.022. Epub 2012 Aug 22. Vaccine. 2012. PMID: 22921739 Free PMC article. Clinical Trial.
-
Dose sparing and the lack of a dose-response relationship with an influenza vaccine in adult and elderly patients - a randomized, double-blind clinical trial.Br J Clin Pharmacol. 2017 Sep;83(9):1912-1920. doi: 10.1111/bcp.13289. Epub 2017 Apr 11. Br J Clin Pharmacol. 2017. PMID: 28378403 Free PMC article. Clinical Trial.
-
Evaluation of influenza virus A/H3N2 and B vaccines on the basis of cross-reactivity of postvaccination human serum antibodies against influenza viruses A/H3N2 and B isolated in MDCK cells and embryonated hen eggs.Clin Vaccine Immunol. 2012 Jun;19(6):897-908. doi: 10.1128/CVI.05726-11. Epub 2012 Apr 4. Clin Vaccine Immunol. 2012. PMID: 22492743 Free PMC article.
-
International Consensus (ICON): allergic reactions to vaccines.World Allergy Organ J. 2016 Sep 16;9(1):32. doi: 10.1186/s40413-016-0120-5. eCollection 2016. World Allergy Organ J. 2016. PMID: 27679682 Free PMC article.
References
-
- Centers for Disease Control and Prevention 2011. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb. Mortal. Wkly. Rep. 60: 1128–1132 - PubMed
-
- Engler RJ, et al. 2008. Half- vs full-dose trivalent inactivated influenza vaccine (2004-2005): age, dose, and sex effects on immune responses. Arch. Intern. Med. 168: 2405–2414 - PubMed
-
- European Agency for the Evaluation of Medicinal Products 2007. EMEA/CHMP/BWP/108182/2007: EU recommendations for the seasonal influenza vaccine composition for the season 2007/2008. European Agency for the Evaluation of Medicinal Products, London, United Kingdom.
-
- European Committee for Proprietary Medicinal Products 1997. Note for guidance on harmonization of requirements for influenza vaccines, March 1997 (CPMP/BWP/214/96). European Directoriate for the Quality of Medicines and Health Care, Strasbourg, France
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical