Difference in Th1 and Th17 lymphocyte adhesion to endothelium
- PMID: 22219321
- PMCID: PMC3262911
- DOI: 10.4049/jimmunol.1101647
Difference in Th1 and Th17 lymphocyte adhesion to endothelium
Abstract
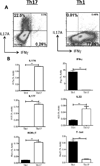
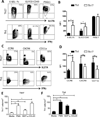
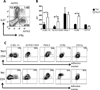
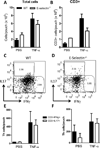
T cell subset-specific migration to inflammatory sites is tightly regulated and involves interaction of the T cells with the endothelium. Th17 cells often appear at different inflammatory sites than Th1 cells, or both subsets appear at the same sites but at different times. Differences in T cell subset adhesion to endothelium may contribute to subset-specific migratory behavior, but this possibility has not been well studied. We examined the adhesion of mouse Th17 cells to endothelial adhesion molecules and endothelium under flow in vitro and to microvessels in vivo and we characterized their migratory phenotype by flow cytometry and quantitative RT-PCR. More Th17 than Th1 cells interacted with E-selectin. Fewer Th17 than Th1 cells bound to TNF-α-activated E-selectin-deficient endothelial cells, and intravital microscopy studies demonstrated that Th17 cells engage in more rolling interactions with TNF-α-treated microvessels than Th1 cells in wild-type mice but not in E-selectin-deficient mice. Th17 adhesion to ICAM-1 was dependent on integrin activation by CCL20, the ligand for CCR6, which is highly expressed by Th17 cells. In an air pouch model of inflammation, CCL20 triggered recruitment of Th17 but not Th1 cells. These data provide evidence that E-selectin- and ICAM-1-dependent adhesion of Th17 and Th1 cells with endothelium are quantitatively different.
Figures


References
-
- Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 2006;18:349–356. - PubMed
-
- Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

