Enhanced HIV-1 neutralization by antibody heteroligation
- PMID: 22219363
- PMCID: PMC3271928
- DOI: 10.1073/pnas.1120059109
Enhanced HIV-1 neutralization by antibody heteroligation
Abstract
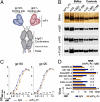
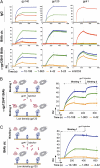
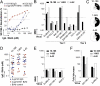
Passive transfer of broadly neutralizing human antibodies against HIV-1 protects macaques against infection. However, HIV-1 uses several strategies to escape antibody neutralization, including mutation of the gp160 viral surface spike, a glycan shield to block antibody access to the spike, and expression of a limited number of viral surface spikes, which interferes with bivalent antibody binding. The latter is thought to decrease antibody apparent affinity or avidity, thereby interfering with neutralizing activity. To test the idea that increasing apparent affinity might enhance neutralizing activity, we engineered bispecific anti-HIV-1 antibodies (BiAbs) that can bind bivalently by virtue of one scFv arm that binds to gp120 and a second arm to the gp41 subunit of gp160. The individual arms of the BiAbs preserved the binding specificities of the original anti-HIV IgG antibodies and together bound simultaneously to gp120 and gp41. Heterotypic bivalent binding enhanced neutralization compared with the parental antibodies. We conclude that antibody recognition and viral neutralization of HIV can be improved by heteroligation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annu Rev Immunol. 2010;28:413–444. - PubMed
-
- Deeks SG, et al. Neutralizing antibody responses against autologous and heterologous viruses in acute versus chronic human immunodeficiency virus (HIV) infection: Evidence for a constraint on the ability of HIV to completely evade neutralizing antibody responses. J Virol. 2006;80:6155–6164. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

