Contact lens physical properties and lipid deposition in a novel characterized artificial tear solution
- PMID: 22219635
- PMCID: PMC3247163
Contact lens physical properties and lipid deposition in a novel characterized artificial tear solution
Abstract
Purpose: To characterize various properties of a physiologically-relevant artificial tear solution (ATS) containing a range of tear film components within a complex salt solution, and to measure contact lens parameters and lipid deposition of a variety of contact lens materials after incubation in this ATS.
Methods: A complex ATS was developed that contains a range of salts, proteins, lipids, mucin, and other tear film constituents in tear-film relevant concentrations. This ATS was tested to confirm that its pH, osmolality, surface tension, and homogeneity are similar to human tears and remain so throughout the material incubation process, for up to 4 weeks. To confirm that silicone hydrogel and conventional hydrogel contact lens materials do not alter in physical characteristics beyond what is allowed by the International Organization for Standardization (ISO) 18369-2. The diameter, center thickness, and calculated base curve were measured for five different lens materials directly out of the blister pack, after a rinse in saline and then following a two week incubation in the modified ATS. To test the ATS and the effect of its composition on lipid deposition, two lens materials were incubated in the ATS and a modified version for several time points. Both ATS solutions contained trace amounts of carbon-14 cholesterol and phosphatidylcholine, such that deposition of these specific lipids could be quantified using standard methods.
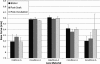
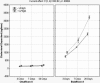
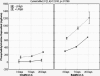
Results: This ATS is a complex mixture that remains stable at physiologically relevant pH (7.3-7.6), osmolality (304-306 mmol/kg), surface tension (40-46 dynes/cm) and homogeneity over an incubation period of three weeks or more. The physical parameters of the lenses tested showed no changes beyond that allowed by the ISO guidelines. Incubations with the ATS found that balafilcon A lenses deposit significantly more cholesterol and phosphatidylcholine than omafilcon A lenses (p<0.05) and that removing lactoferrin and immunoglobulin G from the ATS can significantly decrease the mass of lipid deposited.
Conclusions: This paper describes a novel complex artificial tear solution specially designed for in-vial incubation of contact lens materials. This solution was stable and did not adversely affect the physical parameters of the soft contact lenses incubated within it and showed that lipid deposition was responsive to changes in ATS composition.
Figures
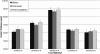
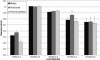
Similar articles
-
Factors that influence in vitro cholesterol deposition on contact lenses.Optom Vis Sci. 2013 Oct;90(10):1057-65. doi: 10.1097/OPX.0000000000000022. Optom Vis Sci. 2013. PMID: 24013797
-
Composition of incubation solution impacts in vitro protein uptake to silicone hydrogel contact lenses.Mol Vis. 2012;18:337-47. Epub 2012 Feb 4. Mol Vis. 2012. PMID: 22355245 Free PMC article.
-
Impact of tear film components on the conformational state of lysozyme deposited on contact lenses.J Biomed Mater Res B Appl Biomater. 2013 Oct;101(7):1172-81. doi: 10.1002/jbm.b.32927. Epub 2013 Apr 6. J Biomed Mater Res B Appl Biomater. 2013. PMID: 23564739
-
Deposition on silicone hydrogel lenses.Eye Contact Lens. 2013 Jan;39(1):20-23. doi: 10.1097/ICL.0b013e318275305b. Eye Contact Lens. 2013. PMID: 23266585 Review.
-
Contact lens interactions with the tear film.Exp Eye Res. 2013 Dec;117:88-98. doi: 10.1016/j.exer.2013.07.013. Epub 2013 Jul 23. Exp Eye Res. 2013. PMID: 23886658 Review.
Cited by
-
Evaluation of ocular tolerability and bioavailability of tonabersat transfersomes ex vivo.Drug Deliv Transl Res. 2025 May 13. doi: 10.1007/s13346-025-01872-2. Online ahead of print. Drug Deliv Transl Res. 2025. PMID: 40358832
-
Differential Deposition of Fluorescently Tagged Cholesterol on Commercial Contact Lenses Using a Novel In Vitro Eye Model.Transl Vis Sci Technol. 2018 Apr 5;7(2):18. doi: 10.1167/tvst.7.2.18. eCollection 2018 Apr. Transl Vis Sci Technol. 2018. PMID: 29644148 Free PMC article.
-
Release of Moxifloxacin from Contact Lenses Using an In Vitro Eye Model: Impact of Artificial Tear Fluid Composition and Mechanical Rubbing.Transl Vis Sci Technol. 2016 Nov 8;5(6):3. doi: 10.1167/tvst.5.6.3. eCollection 2016 Nov. Transl Vis Sci Technol. 2016. PMID: 27847690 Free PMC article.
-
Nanoscale Characteristics of Ocular Lipid Thin Films Using Kelvin Probe Force Microscopy.Transl Vis Sci Technol. 2020 Jun 29;9(7):41. doi: 10.1167/tvst.9.7.41. eCollection 2020 Jun. Transl Vis Sci Technol. 2020. PMID: 32832246 Free PMC article.
-
Development of an In Vitro Blink Model for Ophthalmic Drug Delivery.Pharmaceutics. 2021 Feb 25;13(3):300. doi: 10.3390/pharmaceutics13030300. Pharmaceutics. 2021. PMID: 33668884 Free PMC article.
References
-
- De Smet N, Rymarczyk-Machal M, Schacht E. Modification of polydimethylsiloxane surfaces using benzophenone. J Biomater Sci Polym Ed. 2009;20:2039–53. - PubMed
-
- Fischer M, Sperling C, Werner C. Synergistic effect of hydrophobic and anionic surface groups triggers blood coagulation in vitro. J Mater Sci Mater Med. 2010;21:931–7. - PubMed
-
- Subbiahdoss G, Kuijer R, Busscher HJ, van der Mei HC. Mammalian cell growth versus biofilm formation on biomaterial surfaces in an in vitro post-operative contamination model. Microbiology. 2010;156:3073–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
