Inhibition by female sex hormones of collagen degradation by corneal fibroblasts
- PMID: 22219637
- PMCID: PMC3249437
Inhibition by female sex hormones of collagen degradation by corneal fibroblasts
Abstract
Purpose: Corneal fibroblasts contribute to collagen remodeling in the corneal stroma in part by mediating collagen degradation. Given that corneal structure is influenced by sex hormone status, we examined the effects of sex hormones on collagen degradation by corneal fibroblasts.
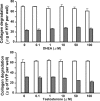
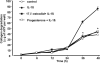
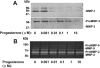
Methods: Rabbit corneal fibroblasts were cultured in three-dimensional collagen gels with or without sex hormones including 17β-estradiol, progesterone, testosterone, and dehydroepiandrosterone (DHEA). Collagen degradation was determined by measurement of hydroxyproline after acid hydrolysis. The expression and activity of matrix metalloproteinases (MMPs) were evaluated by immunoblot analysis and gelatin zymography. The phosphorylation of mitogen-activated protein kinases (MAPKs) and the nuclear factor-kappa B (NF-κB) inhibitor NF kappa B Inhibitor-alpha (IκB-α) in corneal fibroblasts was examined by immunoblot analysis. Cell proliferation and viability were evaluated by measurement of bromodeoxyuridine incorporation and the release of lactate dehydrogenase, respectively.
Results: 17β-Estradiol and progesterone each inhibited interleukin (IL)-1β-induced collagen degradation by corneal fibroblasts in a concentration-dependent manner, whereas testosterone and DHEA had no such effect. MMP expression and activation in corneal fibroblasts exposed to IL-1β were also inhibited by 17β-estradiol and progesterone. These female sex hormones did not affect cell proliferation or viability. Both 17β-estradiol and progesterone inhibited the IL-1β-induced phosphorylation of p38 MAPK without affecting that of the MAPKs extracellular Signal-regulated Kinase (ERK) or c-jun N-terminal kinase (JNK). 17β-Estradiol also inhibited the IL-1β-induced phosphorylation of IκB-α.
Conclusions: 17β-Estradiol and progesterone inhibited MMP expression and activity in IL-1β-stimulated corneal fibroblasts and thereby suppressed collagen degradation by these cells.
Figures


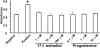



Similar articles
-
Inhibition by medroxyprogesterone acetate of interleukin-1β-induced collagen degradation by corneal fibroblasts.Invest Ophthalmol Vis Sci. 2012 Jun 28;53(7):4213-9. doi: 10.1167/iovs.11-8822. Invest Ophthalmol Vis Sci. 2012. PMID: 22577074
-
Inhibition by a selective IkappaB kinase-2 inhibitor of interleukin-1-induced collagen degradation by corneal fibroblasts in three-dimensional culture.Invest Ophthalmol Vis Sci. 2008 Nov;49(11):4850-7. doi: 10.1167/iovs.08-1897. Epub 2008 Aug 1. Invest Ophthalmol Vis Sci. 2008. PMID: 18676628
-
Dexamethasone inhibition of IL-1-induced collagen degradation by corneal fibroblasts in three-dimensional culture.Invest Ophthalmol Vis Sci. 2004 Sep;45(9):2998-3004. doi: 10.1167/iovs.04-0051. Invest Ophthalmol Vis Sci. 2004. PMID: 15326113
-
Impact of sex in the prevalence and progression of glioblastomas: the role of gonadal steroid hormones.Biol Sex Differ. 2021 Mar 22;12(1):28. doi: 10.1186/s13293-021-00372-5. Biol Sex Differ. 2021. PMID: 33752729 Free PMC article. Review.
-
Gender and the injured brain.Anesth Analg. 2008 Jul;107(1):201-14. doi: 10.1213/ane.0b013e31817326a5. Anesth Analg. 2008. PMID: 18635489 Free PMC article. Review.
Cited by
-
The effect of estrogen and progesterone on porcine corneal biomechanical properties.Graefes Arch Clin Exp Ophthalmol. 2019 Dec;257(12):2691-2695. doi: 10.1007/s00417-019-04490-0. Epub 2019 Oct 17. Graefes Arch Clin Exp Ophthalmol. 2019. PMID: 31624911
-
Differential responses to steroid hormones in fibroblasts from the vocal fold, trachea, and esophagus.Endocrinology. 2015 Mar;156(3):1000-9. doi: 10.1210/en.2014-1605. Epub 2014 Dec 16. Endocrinology. 2015. PMID: 25514085 Free PMC article.
-
Molecular mechanism of the inhibition effect of Lipoxin A4 on corneal dissolving pathology process.Int J Ophthalmol. 2013;6(1):39-43. doi: 10.3980/j.issn.2222-3959.2013.01.08. Epub 2013 Feb 18. Int J Ophthalmol. 2013. PMID: 23550231 Free PMC article.
-
Molecular mechanism of the inhibition effect of Celecoxib on corneal collagen degradation in three dimensions.Int J Ophthalmol. 2012;5(4):434-9. doi: 10.3980/j.issn.2222-3959.2012.04.06. Epub 2012 Aug 18. Int J Ophthalmol. 2012. PMID: 22937501 Free PMC article.
-
Relationship between corneal hysteresis and the site of damage to peripapillary retinal nerve fibre layer thickness in open-angle glaucoma.Sci Rep. 2024 Nov 1;14(1):26329. doi: 10.1038/s41598-024-76187-2. Sci Rep. 2024. PMID: 39487183 Free PMC article.
References
-
- Hoyenga KB, Hoyenga KT. Gender and energy balance: sex differences in adaptations for feast and famine. Physiol Behav. 1982;28:545–63. - PubMed
-
- Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8:523–34. - PubMed
-
- Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Exp Biol Med (Maywood) 2003;228:1175–80. - PubMed
-
- Natoli AK, Medley TL, Ahimastos AA, Drew BG, Thearle DJ, Dilley RJ, Kingwell BA. Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension. 2005;46:1129–34. - PubMed
-
- Ohara N. Sex steroidal modulation of collagen metabolism in uterine leiomyomas. Clin Exp Obstet Gynecol. 2009;36:10–1. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
