Electrochemical microsensors for the detection of cadmium(II) and lead(II) ions in plants
- PMID: 22219663
- PMCID: PMC3247708
- DOI: 10.3390/s100605308
Electrochemical microsensors for the detection of cadmium(II) and lead(II) ions in plants
Abstract
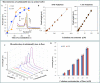
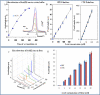
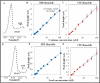
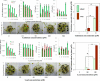
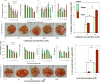
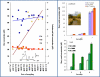
Routine determination of trace metals in complex media is still a difficult task for many analytical instruments. The aim of this work was to compare three electro-chemical instruments [a standard potentiostat (Autolab), a commercially available miniaturized potentiostat (PalmSens) and a homemade micropotentiostat] for easy-to-use and sensitive determination of cadmium(II) and lead(II) ions. The lowest detection limits (hundreds of pM) for both metals was achieved by using of the standard potentiostat, followed by the miniaturized potentiostat (tens of nM) and the homemade instrument (hundreds of nM). Nevertheless, all potentiostats were sensitive enough to evaluate contamination of the environment, because the environmental limits for both metals are higher than detection limits of the instruments. Further, we tested all used potentiostats and working electrodes on analysis of environmental samples (rainwater, flour and plant extract) with artificially added cadmium(II) and lead(II). Based on the similar results obtained for all potentiostats we choose a homemade instrument with a carbon tip working electrode for our subsequent environmental experiments, in which we analyzed maize and sunflower seedlings and rainwater obtained from various sites in the Czech Republic.
Keywords: amperometry; cadmium; heavy metals; lead; maize; miniaturization; plant; screen printed electrode; sunflower; voltammetry; water.
Figures
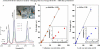


Similar articles
-
Economic bismuth-film microsensor for anodic stripping analysis of trace heavy metals using differential pulse voltammetry.Anal Bioanal Chem. 2005 Nov;383(5):839-47. doi: 10.1007/s00216-005-0083-9. Epub 2005 Nov 5. Anal Bioanal Chem. 2005. PMID: 16215756
-
Highly sensitive determination of cadmium and lead using a low-cost electrochemical flow-through cell based on a carbon paste electrode.Anal Sci. 2012;28(2):141-6. doi: 10.2116/analsci.28.141. Anal Sci. 2012. PMID: 22322806
-
Determination of trace amounts of lead and cadmium using a bismuth/glassy carbon composite electrode.Talanta. 2009 Feb 15;77(4):1432-6. doi: 10.1016/j.talanta.2008.09.028. Epub 2008 Sep 26. Talanta. 2009. PMID: 19084661
-
Modified electrodes used for electrochemical detection of metal ions in environmental analysis.Biosensors (Basel). 2015 Apr 29;5(2):241-75. doi: 10.3390/bios5020241. Biosensors (Basel). 2015. PMID: 25938789 Free PMC article. Review.
-
Conductometric Microbiosensors for Environmental Monitoring.Sensors (Basel). 2008 Apr 11;8(4):2569-2588. doi: 10.3390/s8042569. Sensors (Basel). 2008. PMID: 27879836 Free PMC article. Review.
Cited by
-
Nanosensor Applications in Plant Science.Biosensors (Basel). 2022 Aug 24;12(9):675. doi: 10.3390/bios12090675. Biosensors (Basel). 2022. PMID: 36140060 Free PMC article. Review.
-
Lead ions encapsulated in liposomes and their effect on Staphylococcus aureus.Int J Environ Res Public Health. 2013 Dec 2;10(12):6687-700. doi: 10.3390/ijerph10126687. Int J Environ Res Public Health. 2013. PMID: 24317385 Free PMC article.
-
Voltammetry as a tool for characterization of CdTe quantum dots.Int J Mol Sci. 2013 Jun 27;14(7):13497-510. doi: 10.3390/ijms140713497. Int J Mol Sci. 2013. PMID: 23807507 Free PMC article.
-
Synthesis and Application of Novel Magnetic Ion-Imprinted Polymers for Selective Solid Phase Extraction of Cadmium (II).Polymers (Basel). 2017 Aug 14;9(8):360. doi: 10.3390/polym9080360. Polymers (Basel). 2017. PMID: 30971037 Free PMC article.
-
Recent Advances in the Fabrication and Application of Screen-Printed Electrochemical (Bio)Sensors Based on Carbon Materials for Biomedical, Agri-Food and Environmental Analyses.Biosensors (Basel). 2016 Sep 28;6(4):50. doi: 10.3390/bios6040050. Biosensors (Basel). 2016. PMID: 27690118 Free PMC article. Review.
References
-
- Di Tioppi S.L., Gabbrielli R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999;41:105–130.
-
- Petrek J., Baloun J., Vlasinova H., Havel L., Adam V., Vitecek J., Babula P., Kizek R. Image analysis and activity of intracellular esterases as new analytical tools for determination of growth and viability of embryonic cultures of spruce (Picea sp.) treated with cadmium. Chem. Listy. 2007;101:569–577.
-
- Supalkova V., Huska D., Diopan V., Hanustiak P., Zitka O., Stejskal K., Baloun J., Pikula J., Havel L., Zehnalek J., Adam V., Trnkova L., Beklova M., Kizek R. Electroanalysis of plant thiols. Sensors. 2007;7:932–959.
-
- Supalkova V., Petrek J., Baloun J., Adam V., Bartusek K., Trnkova L., Beklova M., Diopan V., Havel L., Kizek R. Multi-instrumental investigation of affecting of early somatic embryos of spruce by cadmium(II) and lead(II) ions. Sensors. 2007;7:743–759.
-
- Zehnalek J., Adam V., Kizek R. Influence of heavy metals on production of protecting compounds in agriculture plants. Lis. Cukrov. Reparske. 2004;120:222–224.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

