Tris[(1,4,7,10,13,16-hexa-oxacyclo-octa-deca-ne)rubidium] heptaantimonide-ammonia (1/4)
- PMID: 22219792
- PMCID: PMC3246972
- DOI: 10.1107/S1600536811041237
Tris[(1,4,7,10,13,16-hexa-oxacyclo-octa-deca-ne)rubidium] heptaantimonide-ammonia (1/4)
Abstract
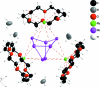
The crystal structure of the title compound, [Rb(C(12)H(24)O(6))](3)[Sb(7)]·4NH(3), fills the gap between the already known Zintl anion ammoniates {[Cs(18-crown-6)](3)Sb(7)}(2)·9NH(3) [Wiesler (2007 ▶). Dissertation, Universität Regensburg, Germany] and [K(18-crown-6)](3)Sb(7)·4NH(3) [Hanauer (2007 ▶). Dissertation, Universität Regensburg, Germany]. As in the two known compounds, the anti-mony cage anion in this crystal structure is coordinated by three alkali cations. The coordination spheres of each of the cations are saturated by 18-crown-6 mol-ecules. The ammonia mol-ecules of crystallization are situated between the crown ethers. The neutral, mol-ecular [Rb(18-crown-6)](3)Sb(7) units are inter-connected by multiple dipole-dipole interactions between ammonia and 18-crown-6.
Figures

References
-
- Adolphson, D. G., Corbett, J. D. & Merryman, D. J. (1976). J. Am. Chem. Soc. 98, 7234–7239.
-
- Brandenburg, K. (2001). DIAMOND Crystal Impact GbR, Bonn, Germany.
-
- Critchlow, S. C. & Corbett, J. D. (1984). Inorg. Chem. 23, 770–774.
-
- Hanauer, T. (2007). Dissertation, Universität Regensburg, Germany.
-
- Hirschle, Ch. & Röhr, C. (2000a). Z. Kristallogr. 17, 164.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
