Methyl (9aR*,10S*,11R*,13aS*,13bS*)-9-oxo-6,7,9,9a,10,11-hexa-hydro-5H,13bH-11,13a-ep-oxy-pyrrolo-[2',1':3,4][1,4]diazepino[2,1-a]isoindole-10-carboxyl-ate
- PMID: 22219895
- PMCID: PMC3247590
- DOI: 10.1107/S1600536811040360
Methyl (9aR*,10S*,11R*,13aS*,13bS*)-9-oxo-6,7,9,9a,10,11-hexa-hydro-5H,13bH-11,13a-ep-oxy-pyrrolo-[2',1':3,4][1,4]diazepino[2,1-a]isoindole-10-carboxyl-ate
Abstract
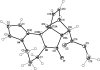
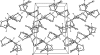
The title compound, C(17)H(18)N(2)O(4), is the methyl ester of the adduct of intra-molecular Diels-Alder reaction between maleic anhydride and 1-(2-fur-yl)-2,3,4,5-tetra-hydro-1H-pyrrolo-[1,2-a][1,4]diazepine. The mol-ecule comprises a fused penta-cyclic system containing four five-membered rings (viz. pyrrole, 2-pyrrolidinone, tetra-hydro-furan and dihydro-furan) and one seven-membered ring (1,4-diazepane). The pyrrole ring is approximately planar (r.m.s. deviation = 0.003 Å) while the 2-pyrrolidinone, tetra-hydro-furan and dihydro-furan five-membered rings have the usual envelope conformations. The central seven-membered diazepane ring adopts a boat conformation. In the crystal, mol-ecules are bound by weak inter-molecular C-H⋯O hydrogen-bonding inter-actions into zigzag chains propagating in [010]. In the crystal packing, the chains are stacked along the a axis.
Figures
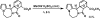
References
-
- Bruker (2001). SAINT-Plus Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Bruker (2005). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Donohoe, T. J. (2000). Science of Synthesis, edited by E. J. Thomas, Vol. 10, p. 653. Stuttgart: Georg Thieme Verlag.
-
- Jones, G. B. & Chapman, B. J. (1996). Comprehensive Heterocyclic Chemistry II, edited by A. R. Katrizky, C. W. Rees & E. F. V. Scriven, Vol. 2, p. 1. Oxford: Pergamon.
-
- Sheldrick, G. M. (2003). SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Research Materials
