1-[(4-{[(2-Oxo-1,2-dihydro-naphthalen-1-yl-idene)meth-yl]amino}-anilino)methyl-idene]naphthalen-2(1H)-one dihydrate
- PMID: 22219984
- PMCID: PMC3247366
- DOI: 10.1107/S1600536811041870
1-[(4-{[(2-Oxo-1,2-dihydro-naphthalen-1-yl-idene)meth-yl]amino}-anilino)methyl-idene]naphthalen-2(1H)-one dihydrate
Abstract
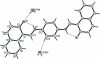
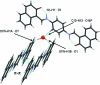
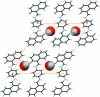
The title compound, C(28)H(20)N(2)O(2)·2H(2)O, comprises a Schiff base mol-ecule with an imposed inversion centre in the middle of p-phenyl-enediamine unit and water mol-ecules of crystallization. In the structure, the Schiff base mol-ecule is present as the keto-amino tautomer with a strong intra-molecular N-H⋯O hydrogen bond. The Schiff base mol-ecules and water mol-ecules of crystallization create infinite [010] columns through O-H⋯O hydrogen bonds. Inter-molecular attractions within columns are through additional π-π inter-actions [centroid-centroid distance = 3.352 (1) Å] between parallel Schiff base mol-ecules. The columns are joined into infinite (011) layers through weak C-H⋯O hydrogen bonds. The layers pack in an assembly by van der Waals attractions, only being effective between bordering non-polar naphthalene ring systems.
Figures

References
-
- Blagus, A., Cinčić, D., Friščić, T., Kaitner, B. & Stilinović, V. (2010). Maced. J. Chem. Chem. Eng. 29, 117–138.
-
- Desiraju, G. R. & Gavezzotti, A. (1989). Acta Cryst. B45, 473–482.
-
- Farrugia, L. J. (1997). J. Appl. Cryst. 30, 565.
-
- Farrugia, L. J. (1999). J. Appl. Cryst. 32, 837–838.
-
- Friščić, T., Kaitner, B. & Meštrović, E. (1998). Croat. Chem. Acta, 71, 87–98.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
