Mitotic spindle orients perpendicular to the forces imposed by dynamic shear
- PMID: 22220200
- PMCID: PMC3248423
- DOI: 10.1371/journal.pone.0028965
Mitotic spindle orients perpendicular to the forces imposed by dynamic shear
Abstract
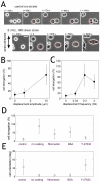
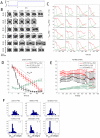
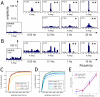
Orientation of the division axis can determine cell fate in the presence of morphogenetic gradients. Understanding how mitotic cells integrate directional cues is therefore an important question in embryogenesis. Here, we investigate the effect of dynamic shear forces on confined mitotic cells. We found that human epithelial cells (hTERT-RPE1) as well as MC3T3 osteoblasts align their mitotic spindle perpendicular to the external force. Spindle orientation appears to be a consequence of cell elongation along the zero-force direction in response to the dynamic shear. This process is a nonlinear response to the strain amplitude, requires actomyosin activity and correlates with redistribution of myosin II. Mechanosteered cells divide normally, suggesting that this mechanism is compatible with biological functions.
Conflict of interest statement
Figures



References
-
- Fishkind DJ, Wang YL. New Horizons for Cytokinesis. Current Opinion in Cell Biology. 1995;7:23–31. - PubMed
-
- Betschinger J, Knoblich JA. Dare to be different: Asymmetric cell division in Drosophila, C-elegans and vertebrates. Current Biology. 2004;14:R674–R685. - PubMed
-
- Strauss B, Adams RJ, Papalopulu N. A default mechanism of spindle orientation based on cell shape is sufficient to generate cell fate diversity in polarised Xenopus blastomeres. Development. 2006;133:3883–3893. - PubMed
-
- Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nature Cell Biology. 2009;11:365–374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

