Triple combination antiviral drug (TCAD) composed of amantadine, oseltamivir, and ribavirin impedes the selection of drug-resistant influenza A virus
- PMID: 22220216
- PMCID: PMC3248427
- DOI: 10.1371/journal.pone.0029778
Triple combination antiviral drug (TCAD) composed of amantadine, oseltamivir, and ribavirin impedes the selection of drug-resistant influenza A virus
Abstract
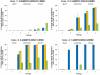
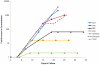
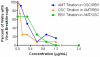
Widespread resistance among circulating influenza A strains to at least one of the anti-influenza drugs is a major public health concern. A triple combination antiviral drug (TCAD) regimen comprised of amantadine, oseltamivir, and ribavirin has been shown to have synergistic and broad spectrum activity against influenza A strains, including drug resistant strains. Here, we used mathematical modeling along with three different experimental approaches to understand the effects of single agents, double combinations, and the TCAD regimen on resistance in influenza in vitro, including: 1) serial passage at constant drug concentrations, 2) serial passage at escalating drug concentrations, and 3) evaluation of the contribution of each component of the TCAD regimen to the suppression of resistance. Consistent with the modeling which demonstrated that three drugs were required to suppress the emergence of resistance in influenza A, treatment with the TCAD regimen resulted in the sustained suppression of drug resistant viruses, whereas treatment with amantadine alone or the amantadine-oseltamivir double combination led to the rapid selection of resistant variants which comprised ∼100% of the population. Furthermore, the TCAD regimen imposed a high genetic barrier to resistance, requiring multiple mutations in order to escape the effects of all the drugs in the regimen. Finally, we demonstrate that each drug in the TCAD regimen made a significant contribution to the suppression of virus breakthrough and resistance at clinically achievable concentrations. Taken together, these data demonstrate that the TCAD regimen was superior to double combinations and single agents at suppressing resistance, and that three drugs at a minimum were required to impede the selection of drug resistant variants in influenza A virus. The use of mathematical modeling with multiple experimental designs and molecular readouts to evaluate and optimize combination drug regimens for the suppression of resistance may be broadly applicable to other infectious diseases.
Conflict of interest statement
Figures

Similar articles
-
Triple combination of amantadine, ribavirin, and oseltamivir is highly active and synergistic against drug resistant influenza virus strains in vitro.PLoS One. 2010 Feb 22;5(2):e9332. doi: 10.1371/journal.pone.0009332. PLoS One. 2010. PMID: 20179772 Free PMC article.
-
Efficacy of combined therapy with amantadine, oseltamivir, and ribavirin in vivo against susceptible and amantadine-resistant influenza A viruses.PLoS One. 2012;7(1):e31006. doi: 10.1371/journal.pone.0031006. Epub 2012 Jan 23. PLoS One. 2012. PMID: 22292088 Free PMC article.
-
Triple combination of oseltamivir, amantadine, and ribavirin displays synergistic activity against multiple influenza virus strains in vitro.Antimicrob Agents Chemother. 2009 Oct;53(10):4115-26. doi: 10.1128/AAC.00476-09. Epub 2009 Jul 20. Antimicrob Agents Chemother. 2009. PMID: 19620324 Free PMC article.
-
Emergence of amantadine-resistant influenza A viruses: epidemiological study.J Infect Chemother. 2003 Sep;9(3):195-200. doi: 10.1007/s10156-003-0262-6. J Infect Chemother. 2003. PMID: 14513385 Review.
-
Antiviral drugs for viruses other than human immunodeficiency virus.Mayo Clin Proc. 2011 Oct;86(10):1009-26. doi: 10.4065/mcp.2011.0309. Mayo Clin Proc. 2011. PMID: 21964179 Free PMC article. Review.
Cited by
-
Combination antiviral therapy for influenza: predictions from modeling of human infections.J Infect Dis. 2012 Jun;205(11):1642-5. doi: 10.1093/infdis/jis265. Epub 2012 Mar 23. J Infect Dis. 2012. PMID: 22448006 Free PMC article.
-
Effective lethal mutagenesis of influenza virus by three nucleoside analogs.J Virol. 2015 Apr;89(7):3584-97. doi: 10.1128/JVI.03483-14. Epub 2015 Jan 14. J Virol. 2015. PMID: 25589650 Free PMC article.
-
Antiviral Potential of Natural Resources against Influenza Virus Infections.Viruses. 2022 Nov 5;14(11):2452. doi: 10.3390/v14112452. Viruses. 2022. PMID: 36366550 Free PMC article. Review.
-
Influenza prevention and treatment in transplant recipients and immunocompromised hosts.Influenza Other Respir Viruses. 2013 Nov;7 Suppl 3(Suppl 3):60-6. doi: 10.1111/irv.12170. Influenza Other Respir Viruses. 2013. PMID: 24215383 Free PMC article. Review.
-
Oseltamivir (Tamiflu(®)) in the environment, resistance development in influenza A viruses of dabbling ducks and the risk of transmission of an oseltamivir-resistant virus to humans - a review.Infect Ecol Epidemiol. 2012;2. doi: 10.3402/iee.v2i0.18385. Epub 2012 Jun 21. Infect Ecol Epidemiol. 2012. PMID: 22957124 Free PMC article.
References
-
- Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. - PubMed
-
- Daar ES, Richman DD. Confronting the emergence of drug-resistant HIV type 1: impact of antiretroviral therapy on individual and population resistance. AIDS Res Hum Retroviruses. 2005;21:343–357. - PubMed
-
- Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. - PubMed
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

