Pioglitazone treatment increases COX-2-derived prostacyclin production and reduces oxidative stress in hypertensive rats: role in vascular function
- PMID: 22220498
- PMCID: PMC3417448
- DOI: 10.1111/j.1476-5381.2012.01825.x
Pioglitazone treatment increases COX-2-derived prostacyclin production and reduces oxidative stress in hypertensive rats: role in vascular function
Abstract
Background and purpose: PPARγ agonists, glitazones, have cardioprotective and anti-inflammatory actions associated with gene transcription interference. In this study, we determined whether chronic treatment of adult spontaneously hypertensive rats (SHR) with pioglitazone alters BP and vascular structure and function, and the possible mechanisms involved.
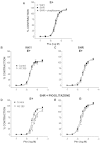
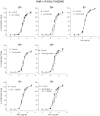
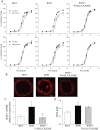
Experimental approach: Mesenteric resistance arteries from untreated or pioglitazone-treated (2.5 mg·kg⁻¹ ·day⁻¹ , 28 days) SHR and normotensive [Wistar Kyoto (WKY)] rats were used. Vascular structure was studied by pressure myography, vascular function by wire myography, protein expression by Western blot and immunohistochemistry, mRNA levels by RT-PCR, prostanoid levels by commercial kits and reactive oxygen species (ROS) production by dihydroethidium-emitted fluorescence.
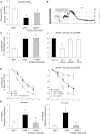
Key results: In SHR, pioglitazone did not modify either BP or vascular structural and mechanical alterations or phenylephrine-induced contraction, but it increased vascular COX-2 levels, prostacyclin (PGI₂) production and the inhibitory effects of NS 398, SQ 29,548 and tranylcypromine on phenylephrine responses. The contractile phase of the iloprost response, which was reduced by SQ 29,548, was greater in pioglitazone-treated and pioglitazone-untreated SHR than WKY. In addition, pioglitazone abolished the increased vascular ROS production, NOX-1 levels and the inhibitory effect of apocynin and allopurinol on phenylephrine contraction, whereas it did not modify eNOS expression but restored the potentiating effect of N-nitro-L-arginine methyl ester on phenylephrine responses.
Conclusions and implications: Although pioglitazone did not reduce BP in SHR, it increased COX-2-derived PGI₂ production, reduced oxidative stress, and increased NO bioavailability, which are all involved in vasoconstrictor responses in resistance arteries. These effects would contribute to the cardioprotective effect of glitazones reported in several pathologies.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures

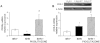
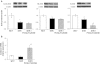

Similar articles
-
Atorvastatin prevents endothelial dysfunction in mesenteric arteries from spontaneously hypertensive rats: role of cyclooxygenase 2-derived contracting prostanoids.Hypertension. 2009 Jun;53(6):1008-16. doi: 10.1161/HYPERTENSIONAHA.109.132258. Epub 2009 Apr 20. Hypertension. 2009. PMID: 19380610
-
Peroxisome proliferator-activated receptor-γ activation reduces cyclooxygenase-2 expression in vascular smooth muscle cells from hypertensive rats by interfering with oxidative stress.J Hypertens. 2012 Feb;30(2):315-26. doi: 10.1097/HJH.0b013e32834f043b. J Hypertens. 2012. PMID: 22179086
-
Reciprocal relationship between reactive oxygen species and cyclooxygenase-2 and vascular dysfunction in hypertension.Antioxid Redox Signal. 2013 Jan 1;18(1):51-65. doi: 10.1089/ars.2011.4335. Epub 2012 Jul 19. Antioxid Redox Signal. 2013. PMID: 22671943
-
Losartan reduces the increased participation of cyclooxygenase-2-derived products in vascular responses of hypertensive rats.J Pharmacol Exp Ther. 2007 Apr;321(1):381-8. doi: 10.1124/jpet.106.115287. Epub 2007 Jan 23. J Pharmacol Exp Ther. 2007. PMID: 17244722
-
Neurogenic hypertension: revelations from genome-wide gene expression profiling.Curr Hypertens Rep. 2012 Dec;14(6):485-91. doi: 10.1007/s11906-012-0282-7. Curr Hypertens Rep. 2012. PMID: 22639016 Review.
Cited by
-
Endothelial Dysfunction and Passive Changes in the Aorta and Coronary Arteries of Diabetic db/db Mice.Front Physiol. 2020 Jun 23;11:667. doi: 10.3389/fphys.2020.00667. eCollection 2020. Front Physiol. 2020. PMID: 32655412 Free PMC article.
-
Mitochondria and cardiovascular diseases-from pathophysiology to treatment.Ann Transl Med. 2018 Jun;6(12):256. doi: 10.21037/atm.2018.06.21. Ann Transl Med. 2018. PMID: 30069458 Free PMC article. Review.
-
Thiazolidinediones improve flow-mediated dilation: a meta-analysis of randomized clinical trials.Eur J Clin Pharmacol. 2016 Apr;72(4):385-98. doi: 10.1007/s00228-015-1999-4. Epub 2015 Dec 22. Eur J Clin Pharmacol. 2016. PMID: 26690770 Review.
-
Biliary atresia susceptibility gene EFEMP1 regulates extrahepatic bile duct elastic fiber formation and mechanics.JHEP Rep. 2024 Sep 8;7(1):101215. doi: 10.1016/j.jhepr.2024.101215. eCollection 2025 Jan. JHEP Rep. 2024. PMID: 39717503 Free PMC article.
-
Peroxisome proliferator-activated receptors and Alzheimer's disease: hitting the blood-brain barrier.Mol Neurobiol. 2013 Dec;48(3):438-51. doi: 10.1007/s12035-013-8435-5. Epub 2013 Mar 14. Mol Neurobiol. 2013. PMID: 23494748 Review.
References
-
- Alvarez Y, Briones AM, Balfagón G, Alonso MJ, Salaices M. Hypertension increases the participation of vasoconstrictor prostanoids from cyclooxygenase-2 in phenylephrine responses. J Hypertens. 2005;23:767–777. - PubMed
-
- Alvarez Y, Pérez-Girón JV, Hernanz R, Briones AM, García-Redondo A, Beltrán A, et al. Losartan reduces the increased participation of cyclooxygenase-2-derived products in vascular responses of hypertensive rats. J Pharmacol Exp Ther. 2007;321:381–388. - PubMed
-
- Arribas SM, Hillier C, González C, McGrory S, Dominiczak AF, McGrath JC. Cellular aspects of vascular remodeling in hypertension revealed by confocal microscopy. Hypertension. 1997;30:1455–1464. - PubMed
-
- Bagi Z, Koller A, Kaley G. PPARγ activation, by reducing oxidative stress, increases NO bioavailability in coronary arterioles of mice with Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2004;286:H742–H748. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

