Lysosomal signaling enhances mitochondria-mediated photodynamic therapy in A431 cancer cells: role of iron
- PMID: 22220628
- PMCID: PMC4547857
- DOI: 10.1111/j.1751-1097.2012.01081.x
Lysosomal signaling enhances mitochondria-mediated photodynamic therapy in A431 cancer cells: role of iron
Abstract
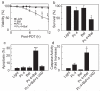
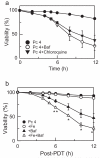
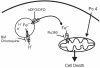
In photodynamic therapy (PDT), light activates a photosensitizer added to a tissue, resulting in singlet oxygen formation and cell death. The photosensitizer phthalocyanine 4 (Pc 4) localizes primarily to mitochondrial membranes in cancer cells, resulting in mitochondria-mediated cell death. The aim of this study was to determine how lysosomes contribute to PDT-induced cell killing by mitochondria-targeted photosensitizers such as Pc 4. We monitored cell killing of A431 cells after Pc 4-PDT in the presence and absence of bafilomycin, an inhibitor of the vacuolar proton pump of lysosomes and endosomes. Bafilomycin was not toxic by itself, but greatly enhanced Pc 4-PDT-induced cell killing. To investigate whether iron loading of lysosomes affects bafilomycin-induced killing, cells were incubated with ammonium ferric citrate (30 μM) for 30 h prior to PDT. Ammonium ferric citrate enhanced Pc 4 plus bafilomycin-induced cell killing without having toxicity by itself. Iron chelators (desferrioxamine and starch-desferrioxamine) and the inhibitor of the mitochondrial calcium (and ferrous iron) uniporter, Ru360, protected against Pc 4 plus bafilomycin toxicity. These results support the conclusion that chelatable iron stored in the lysosomes enhances the efficacy of bafilomycin-mediated PDT and that lysosomal disruption augments PDT with Pc 4.
© 2012 Wiley Periodicals, Inc. Photochemistry and Photobiology © 2012 The American Society of Photobiology.
Figures




Similar articles
-
Mitoferrin-2-dependent mitochondrial iron uptake sensitizes human head and neck squamous carcinoma cells to photodynamic therapy.J Biol Chem. 2013 Jan 4;288(1):677-86. doi: 10.1074/jbc.M112.422667. Epub 2012 Nov 7. J Biol Chem. 2013. PMID: 23135267 Free PMC article.
-
Signaling From Lysosomes Enhances Mitochondria-Mediated Photodynamic Therapy In Cancer Cells.Proc SPIE Int Soc Opt Eng. 2009 Jul 12;7380(73800C):1-8. doi: 10.1117/12.823752. Proc SPIE Int Soc Opt Eng. 2009. PMID: 20228965 Free PMC article.
-
Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury.Hepatology. 2008 Nov;48(5):1644-54. doi: 10.1002/hep.22498. Hepatology. 2008. PMID: 18846543 Free PMC article.
-
Signaling pathways in cell death and survival after photodynamic therapy.J Photochem Photobiol B. 2000 Aug;57(1):1-13. doi: 10.1016/s1011-1344(00)00065-8. J Photochem Photobiol B. 2000. PMID: 11100832 Review.
-
Mitochondria-based photodynamic anti-cancer therapy.Adv Drug Deliv Rev. 2001 Jul 2;49(1-2):71-86. doi: 10.1016/s0169-409x(01)00126-0. Adv Drug Deliv Rev. 2001. PMID: 11377804 Review.
Cited by
-
Apoptosis and associated phenomena as a determinants of the efficacy of photodynamic therapy.Photochem Photobiol Sci. 2015 Aug;14(8):1397-402. doi: 10.1039/c4pp00413b. Epub 2015 Jan 5. Photochem Photobiol Sci. 2015. PMID: 25559971 Free PMC article. Review.
-
Photodynamic Efficiency: From Molecular Photochemistry to Cell Death.Int J Mol Sci. 2015 Aug 31;16(9):20523-59. doi: 10.3390/ijms160920523. Int J Mol Sci. 2015. PMID: 26334268 Free PMC article. Review.
-
Photosensitizer adhered to cell culture microplates induces phototoxicity in carcinoma cells.Biomed Res Int. 2013;2013:549498. doi: 10.1155/2013/549498. Epub 2012 Dec 23. Biomed Res Int. 2013. PMID: 23509741 Free PMC article.
-
Promotion of Proapoptotic Signals by Lysosomal Photodamage.Photochem Photobiol. 2015 Jul-Aug;91(4):931-6. doi: 10.1111/php.12456. Epub 2015 May 8. Photochem Photobiol. 2015. PMID: 25873082 Free PMC article.
-
Natural Products Induce Lysosomal Membrane Permeabilization as an Anticancer Strategy.Medicines (Basel). 2021 Nov 10;8(11):69. doi: 10.3390/medicines8110069. Medicines (Basel). 2021. PMID: 34822366 Free PMC article. Review.
References
-
- Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem. Photobiol. Sci. 2002;1:1–21. - PubMed
-
- Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J. Biol. Chem. 2001;276:47379–47386. - PubMed
-
- Trivedi NS, Wang HW, Nieminen AL, Oleinick NL, Izatt JA. Quantitative analysis of Pc 4 localization in mouse lymphoma (LY-R) cells via double-label confocal fluorescence microscopy. Photochem. Photobiol. 2000;71:634–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

