Epigenetic dysregulation of the dopamine system in diet-induced obesity
- PMID: 22220805
- PMCID: PMC3296832
- DOI: 10.1111/j.1471-4159.2012.07649.x
Epigenetic dysregulation of the dopamine system in diet-induced obesity
Abstract
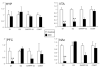
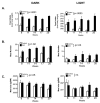
Chronic intake of high-fat (HF) diet is known to alter brain neurotransmitter systems that participate in the central regulation of food intake. Dopamine (DA) system changes in response to HF diet have been observed in the hypothalamus, important in the homeostatic control of food intake, as well as within the central reward circuitry [ventral tegmental area (VTA), nucleus accumbens (NAc), and pre-frontal cortex (PFC)], critical for coding the rewarding properties of palatable food and important in hedonically driven feeding behavior. Using a mouse model of diet-induced obesity (DIO), significant alterations in the expression of DA-related genes were documented in adult animals, and the general pattern of gene expression changes was opposite within the hypothalamus versus the reward circuitry (increased vs. decreased, respectively). Differential DNA methylation was identified within the promoter regions of tyrosine hydroxylase (TH) and dopamine transporter (DAT), and the pattern of this response was consistent with the pattern of gene expression. Behaviors consistent with increased hypothalamic DA and decreased reward circuitry DA were observed. These data identify differential DNA methylation as an epigenetic mechanism linking the chronic intake of HF diet with altered DA-related gene expression, and this response varies by brain region and DNA sequence.
© 2012 The Authors. Journal of Neurochemistry © 2012 International Society for Neurochemistry.
Figures

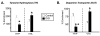

Similar articles
-
Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes.Endocrinology. 2010 Oct;151(10):4756-64. doi: 10.1210/en.2010-0505. Epub 2010 Aug 4. Endocrinology. 2010. PMID: 20685869 Free PMC article.
-
Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet.Biol Psychiatry. 2008 Dec 1;64(11):941-50. doi: 10.1016/j.biopsych.2008.06.007. Epub 2008 Jul 26. Biol Psychiatry. 2008. PMID: 18657800 Free PMC article.
-
Reversal of dopamine system dysfunction in response to high-fat diet.Obesity (Silver Spring). 2013 Dec;21(12):2513-21. doi: 10.1002/oby.20374. Epub 2013 May 29. Obesity (Silver Spring). 2013. PMID: 23512420 Free PMC article.
-
Dopamine-based reward circuitry responsivity, genetics, and overeating.Curr Top Behav Neurosci. 2011;6:81-93. doi: 10.1007/7854_2010_89. Curr Top Behav Neurosci. 2011. PMID: 21243471 Review.
-
Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity.Neuroimage Clin. 2015 Mar 24;8:1-31. doi: 10.1016/j.nicl.2015.03.016. eCollection 2015. Neuroimage Clin. 2015. PMID: 26110109 Free PMC article. Review.
Cited by
-
Gestational overgrowth and undergrowth affect neurodevelopment: similarities and differences from behavior to epigenetics.Int J Dev Neurosci. 2013 Oct;31(6):406-14. doi: 10.1016/j.ijdevneu.2012.11.006. Epub 2012 Nov 28. Int J Dev Neurosci. 2013. PMID: 23201144 Free PMC article. Review.
-
Animal Models and Their Contribution to Our Understanding of the Relationship Between Environments, Epigenetic Modifications, and Behavior.Genes (Basel). 2019 Jan 15;10(1):47. doi: 10.3390/genes10010047. Genes (Basel). 2019. PMID: 30650619 Free PMC article. Review.
-
Dopamine gene methylation patterns are associated with obesity markers and carbohydrate intake.Brain Behav. 2018 Aug;8(8):e01017. doi: 10.1002/brb3.1017. Epub 2018 Jul 11. Brain Behav. 2018. PMID: 29998543 Free PMC article.
-
Chronic consumption of a western diet modifies the DNA methylation profile in the frontal cortex of mice.Food Funct. 2018 Feb 21;9(2):1187-1198. doi: 10.1039/c7fo01602f. Food Funct. 2018. PMID: 29372223 Free PMC article.
-
Modulation of taste responsiveness and food preference by obesity and weight loss.Physiol Behav. 2012 Nov 5;107(4):527-32. doi: 10.1016/j.physbeh.2012.04.004. Epub 2012 Apr 12. Physiol Behav. 2012. PMID: 22521912 Free PMC article. Review.
References
-
- Alsiö J, Olszewski PK, Norbäck AH, Gunnarsson ZE, Levine AS, Pickering C, Schiöth HB. Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience. 2010;171:779–787. - PubMed
-
- Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. - PubMed
-
- Briggs DI, Enriori PJ, Lemus MB, Cowley MA, Andrews ZB. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology. 2010;151:10. - PubMed
-
- Chen JC, Turiak G, Galler J, Volicer L. Postnatal changes of brain monoamine levels in prenatally malnourished and control rats. Int J Dev Neurosci. 1997;15:527–263. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

