The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities
- PMID: 22221091
- PMCID: PMC3276249
- DOI: 10.1111/j.1365-2958.2012.07968.x
The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities
Abstract
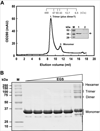
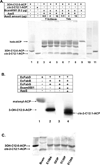
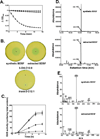
Signal molecules of the diffusible signal factor (DSF) family have been shown recently to be involved in regulation of pathogenesis and biofilm formation in diverse Gram-negative bacteria. DSF signals are reported to be active not only on their cognate bacteria, but also on unrelated bacteria and the pathogenic yeast, Candida albicans. DSFs are monounsaturated fatty acids of medium chain length containing an unusual cis-2 double bond. Although genetic analyses had identified genes involved in DSF synthesis, the pathway of DSF synthesis was unknown. The DSF of the important human pathogen Burkholderia cenocepacia (called BDSF) is cis-2-dodecenoic acid. We report that BDSF is synthesized from a fatty acid synthetic intermediate, the acyl carrier protein (ACP) thioester of 3-hydroxydodecanoic acid. This intermediate is intercepted by protein Bcam0581 and converted to cis-2-dodecenoyl-ACP. Bcam0581 is annotated as a homologue of crotonase, the first enzyme of the fatty acid degradation pathway. We demonstrated Bcam0581to be a bifunctional protein that not only catalysed dehydration of 3-hydroxydodecanoyl-ACP to cis-2-dodecenoyl-ACP, but also cleaved the thioester bond to give the free acid. Both activities required the same set of active-site residues. Although dehydratase and thioesterase activities are known activities of the crotonase superfamily, Bcam0581 is the first protein shown to have both activities.
© 2012 Blackwell Publishing Ltd.
Figures




References
-
- Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJ, Slater H, Dow JM, Williams P, Daniels MJ. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol. 1997;24:555–566. - PubMed
-
- Batchelar ET, Hamed RB, Ducho C, Claridge TD, Edelmann MJ, Kessler B, Schofield CJ. Thioester hydrolysis and C-C bond formation by carboxymethylproline synthase from the crotonase superfamily. Angew Chem Int Ed Engl. 2008;47:9322–9325. - PubMed
-
- Boon C, Deng Y, Wang LH, He Y, Xu JL, Fan Y, Pan SQ, Zhang LH. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2008;2:27–36. - PubMed
-
- Cheng Z, He YW, Lim SC, Qamra R, Walsh MA, Zhang LH, Song H. Structural basis of the sensor-synthase interaction in autoinduction of the quorum sensing signal DSF biosynthesis. Structure. 2010;18:1199–1209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

