Homocysteine impairs endothelial wound healing by activating metabotropic glutamate receptor 5
- PMID: 22221504
- PMCID: PMC6359906
- DOI: 10.1111/j.1549-8719.2012.00159.x
Homocysteine impairs endothelial wound healing by activating metabotropic glutamate receptor 5
Erratum in
- Microcirculation. 2012 Jul;19(5):472
Abstract
Objective: Hcy is an independent risk factor for cerebrovascular disease and cognitive impairment. The purpose of this study was to elucidate the role of mGluR5 in Hcy-mediated impairment of cerebral endothelial wound repair.
Methods: Mouse CMVECs (bEnd.3) were used in conjunction with directed pharmacology and shRNA. AutoDock was used to simulate the docking of ligand-receptor interactions.
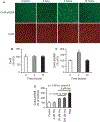
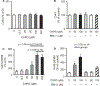
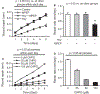
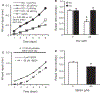
Results: Hcy (20 μM) significantly increased Cx43-pS368 by mGluR5- and PKC-dependent mechanisms. Hcy attenuated wound repair by an mGluR5-dependent mechanism over the six-day study period but did not alter cell proliferation in a proliferation assay, suggesting that the attenuation of wound repair may be due to dysfunctional migration in HHcy. Hcy increased the expression of Cx43 and Cx43-pS368 at the wound edge by activating mGluR5. Direct activation of mGluR5, using the specific agonist CHPG, was sufficient to reproduce the results whereas KO of mGluR5 with shRNA, or inhibition with MPEP, blocked the response to Hcy.
Conclusions: Inhibition of mGluR5 activation could be a novel strategy for promoting endothelial wound repair in patients with HHcy. Activation of mGluR5 may be a viable strategy for disrupting angiogenesis.
© 2012 John Wiley & Sons Ltd.
Figures




Similar articles
-
Metabotropic glutamate receptor 5 mediates phosphorylation of vascular endothelial cadherin and nuclear localization of β-catenin in response to homocysteine.Vascul Pharmacol. 2012 Mar-Apr;56(3-4):159-67. doi: 10.1016/j.vph.2012.01.004. Epub 2012 Jan 21. Vascul Pharmacol. 2012. PMID: 22285407 Free PMC article.
-
Nitrative stress in cerebral endothelium is mediated by mGluR5 in hyperhomocysteinemia.J Cereb Blood Flow Metab. 2012 May;32(5):825-34. doi: 10.1038/jcbfm.2011.185. Epub 2011 Dec 21. J Cereb Blood Flow Metab. 2012. PMID: 22186670 Free PMC article.
-
The glutamate agonist homocysteine sulfinic acid stimulates glucose uptake through the calcium-dependent AMPK-p38 MAPK-protein kinase C zeta pathway in skeletal muscle cells.J Biol Chem. 2011 Mar 4;286(9):7567-76. doi: 10.1074/jbc.M110.149328. Epub 2010 Dec 30. J Biol Chem. 2011. PMID: 21193401 Free PMC article.
-
Metabotropic glutamate receptor 5 in the pathology and treatment of schizophrenia.Neurosci Biobehav Rev. 2013 Mar;37(3):256-68. doi: 10.1016/j.neubiorev.2012.12.005. Epub 2012 Dec 17. Neurosci Biobehav Rev. 2013. PMID: 23253944 Review.
-
The role of metabotropic glutamate receptor 5 in learning and memory processes.Drug News Perspect. 2005 Jul-Aug;18(6):353-61. doi: 10.1358/dnp.2005.18.6.927927. Drug News Perspect. 2005. PMID: 16247513 Review.
Cited by
-
The connexin 43/ZO-1 complex regulates cerebral endothelial F-actin architecture and migration.Am J Physiol Cell Physiol. 2015 Nov 1;309(9):C600-7. doi: 10.1152/ajpcell.00155.2015. Epub 2015 Aug 19. Am J Physiol Cell Physiol. 2015. PMID: 26289751 Free PMC article.
-
Homocysteine disrupts outgrowth of microvascular endothelium by an iNOS-dependent mechanism.Microcirculation. 2014 Aug;21(6):541-50. doi: 10.1111/micc.12133. Microcirculation. 2014. PMID: 24655004 Free PMC article.
-
Regulatory Roles of Metabotropic Glutamate Receptors on Synaptic Communication Mediated by Gap Junctions.Neuroscience. 2021 Feb 21;456:85-94. doi: 10.1016/j.neuroscience.2020.06.034. Epub 2020 Jun 30. Neuroscience. 2021. PMID: 32619474 Free PMC article. Review.
-
Homocysteine metabolism as the target for predictive medical approach, disease prevention, prognosis, and treatments tailored to the person.EPMA J. 2021 Nov 11;12(4):477-505. doi: 10.1007/s13167-021-00263-0. eCollection 2021 Dec. EPMA J. 2021. PMID: 34786033 Free PMC article. Review.
-
Phyllanthus emblica L. Enhances Human Umbilical Vein Endothelial Wound Healing and Sprouting.Evid Based Complement Alternat Med. 2013;2013:720728. doi: 10.1155/2013/720728. Epub 2013 Mar 31. Evid Based Complement Alternat Med. 2013. PMID: 23606890 Free PMC article.
References
-
- Brauner-Osborne H, Wellendorph P, Jensen AA. Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets 8: 169–184, 2007. - PubMed
-
- Carmeliet P Angiogenesis in life, disease and medicine. Nature 438: 932–936, 2005. - PubMed
-
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol 1: 92–100, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

