Modulation of microcirculatory changes in the late phase of hepatic ischaemia-reperfusion injury by remote ischaemic preconditioning
- PMID: 22221569
- PMCID: PMC3277050
- DOI: 10.1111/j.1477-2574.2011.00407.x
Modulation of microcirculatory changes in the late phase of hepatic ischaemia-reperfusion injury by remote ischaemic preconditioning
Abstract
Background: Remote ischaemic preconditioning (RIPC) is a novel method of protecting the liver from ischaemia-reperfusion (I-R) injury. Protective effects in the early phase (4-6 h) have been demonstrated, but no studies have focused on the late phase (24 h) of hepatic I-R. This study analysed events in the late phase of I-R following RIPC and focused on the microcirculation, inflammatory cascade and the role of cytokine-induced neutrophil chemoattractant-1 (CINC-1).
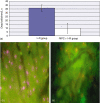
Methods: A standard animal model was used. Remote preconditioning prior to I-R was induced by intermittent limb ischaemia. Ischaemia was induced in the left and median lobes of the liver (70%). The animals were recovered after 45 min of liver ischaemia. At 24 h, the animals were re-evaluated under anaesthesia. Hepatic microcirculation, sinusoidal leukocyte adherence and hepatocellular death were assessed by intravital microscopy, hepatocellular injury by standard biochemistry and serum CINC-1 by enzyme-linked immunosorbent assay (ELISA).
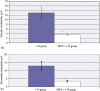
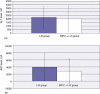
Results: At 24 h post I-R, RIPC was found to have improved sinusoidal flow by increasing the sinusoidal diameter. There was no effect of preconditioning on the velocity of red blood cells, by contrast with the early phase of hepatic I-R. Remote ischaemic preconditioning significantly reduced hepatocellular injury, neutrophil-induced endothelial injury and serum CINC-1 levels.
Conclusions: Remote ischaemic preconditioning is amenable to translation into clinical practice and may improve outcomes in liver resection surgery and transplantation.
© 2011 International Hepato-Pancreato-Biliary Association.
Figures







References
-
- Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829–838. - PubMed
-
- Jaeschke H, Farhood A, Smith CW. Neutrophils contribute to ischaemia/reperfusion injury in rat liver in vivo. FASEB J. 1990;4:3355–3359. - PubMed
-
- Jassem W, Fuggle SV, Cerundolo L, Heaton ND, Rela M. Ischaemic preconditioning of cadaver donor livers protects allografts following transplantation. Transplantation. 2006;81:169–174. - PubMed
-
- Peralta C, Fernandez L, Panes J, Prats N, Sans M, Pique JM, et al. Preconditioning protects against systemic disorders associated with hepatic ischaemia–reperfusion through blockade of tumour necrosis factor-induced P-selectin upregulation in the rat. Hepatology. 2001;33:100–113. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

