Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial
- PMID: 22221594
- PMCID: PMC3331916
- DOI: 10.1111/j.1360-0443.2011.03771.x
Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial
Erratum in
- Addiction. 2012 Sep;107(9):1718. Dosage error in published abstract; MEDLINE/PubMed abstract corrected
Abstract
Aims: Topiramate has shown efficacy at facilitating abstinence from alcohol and cocaine abuse. This double-blind, placebo-controlled out-patient trial tested topiramate for treating methamphetamine addiction.
Design: Participants (n = 140) were randomized to receive topiramate or placebo (13 weeks) in escalating doses from 25 mg/day [DOSAGE ERROR CORRECTED] to the target maintenance of 200 mg/day in weeks 6-12 (tapered in week 13). Medication was combined with weekly brief behavioral compliance enhancement treatment.
Setting: The trial was conducted at eight medical centers in the United States.
Participants: One hundred and forty methamphetamine-dependent adults took part in the trial.
Measurements: The primary outcome was abstinence from methamphetamine during weeks 6-12. Secondary outcomes included use reduction versus baseline, as well as psychosocial variables.
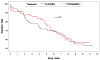
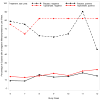
Findings: In the intent-to-treat analysis, topiramate did not increase abstinence from methamphetamine during weeks 6-12. For secondary outcomes, topiramate reduced weekly median urine methamphetamine levels and observer-rated severity of dependence scores significantly. Subjects with negative urine before randomization (n = 26) had significantly greater abstinence on topiramate versus placebo during study weeks 6-12. Topiramate was safe and well tolerated.
Conclusions: Topiramate does not appear to promote abstinence in methamphetamine users but can reduce the amount taken and reduce relapse rates in those who are already abstinent.
Trial registration: ClinicalTrials.gov NCT00345371.
© 2011 The Authors, Addiction © 2011 Society for the Study of Addiction.
Conflict of interest statement
Figures


Comment in
-
Commentary on Elkashef et al. (2012): just enough efficacy for a second look.Addiction. 2012 Jul;107(7):1307-8. doi: 10.1111/j.1360-0443.2012.03841.x. Addiction. 2012. PMID: 22672376 No abstract available.
References
-
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. - PubMed
-
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. - PubMed
-
- Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend. 2004;75:233–240. - PubMed
-
- Kushner SA, Dewey SL, Kornetsky C. The irreversible gamma-aminobutyric acid (GABA) transaminase inhibitor gamma-vinyl-GABA blocks cocaine self-administration in rats. J Pharmacol Exp Ther. 1999;290:797–802. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

