Distinct recycling of active and inactive β1 integrins
- PMID: 22222055
- PMCID: PMC3531618
- DOI: 10.1111/j.1600-0854.2012.01327.x
Distinct recycling of active and inactive β1 integrins
Abstract
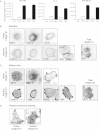
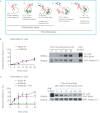
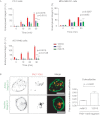
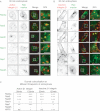
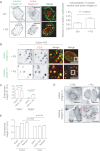
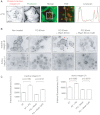
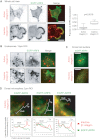
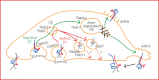
Integrin trafficking plays an important role in cellular motility and cytokinesis. Integrins undergo constant endo/exocytic shuttling to facilitate the dynamic regulation of cell adhesion. Integrin activity toward the components of the extracellular matrix is regulated by the ability of these receptors to switch between active and inactive conformations. Several cellular signalling pathways have been described in the regulation of integrin traffic under different conditions. However, the interrelationship between integrin activity conformations and their endocytic fate have remained incompletely understood. Here, we have investigated the endocytic trafficking of active and inactive β1 integrins in cancer cells. Both conformers are endocytosed in a clathrin- and dynamin-dependent manner. The net endocytosis rate of the active β1 integrins is higher, whereas endocytosis of the inactive β1 integrin is counteracted by rapid recycling back to the plasma membrane via an ARF6- and early endosome antigen 1-positive compartment in an Rab4a- and actin-dependent manner. Owing to these distinct trafficking routes, the two receptor pools display divergent subcellular localization. At steady state, the inactive β1 integrin is mainly on the plasma membrane, whereas the active receptor is predominantly intracellular. These data provide new insights into the endocytic traffic of integrins and imply the possibility of a previously unappreciated crosstalk between pathways regulating integrin activity and traffic.
© 2012 John Wiley & Sons A/S.
Figures


References
-
- Fletcher SJ, Rappoport JZ. Moving forward: polarised trafficking in cell migration. Trends Cell Biol. 2010;20:71–78. - PubMed
-
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
-
- Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

