Muscarinic receptor agonists and antagonists: effects on cancer
- PMID: 22222710
- PMCID: PMC3604886
- DOI: 10.1007/978-3-642-23274-9_19
Muscarinic receptor agonists and antagonists: effects on cancer
Abstract
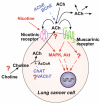
Many epithelial and endothelial cells express a cholinergic autocrine loop in which acetylcholine acts as a growth factor to stimulate cell growth. Cancers derived from these tissues similarly express a cholinergic autocrine loop and ACh secreted by the cancer or neighboring cells interacts with M3 muscarinic receptors expressed on the cancer cells to stimulate tumor growth. Primary proliferative pathways involve MAPK and Akt activation. The ability of muscarinic agonists to stimulate, and M3 antagonists to inhibit tumor growth has clearly been demonstrated for lung and colon cancer. The ability of muscarinic agonists to stimulate growth has been shown for melanoma, pancreatic, breast, ovarian, prostate and brain cancers, suggesting that M3 antagonists will also inhibit growth of these tumors as well. As yet no clinical trials have proven the efficacy of M3 antagonists as cancer therapeutics, though the widespread clinical use and low toxicity of M3 antagonists support the potential role of these drugs as adjuvants to current cancer therapies.
Figures




Similar articles
-
Muscarinic receptor subtypes in neuronal and non-neuronal cholinergic function.Auton Autacoid Pharmacol. 2006 Jul;26(3):219-33. doi: 10.1111/j.1474-8673.2006.00368.x. Auton Autacoid Pharmacol. 2006. PMID: 16879488 Review.
-
Muscarinic receptor agonists and antagonists: effects on inflammation and immunity.Handb Exp Pharmacol. 2012;(208):403-27. doi: 10.1007/978-3-642-23274-9_17. Handb Exp Pharmacol. 2012. PMID: 22222708 Review.
-
Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor.Clin Cancer Res. 1999 Sep;5(9):2532-9. Clin Cancer Res. 1999. PMID: 10499630
-
Muscarinic agonists and antagonists: effects on the urinary bladder.Handb Exp Pharmacol. 2012;(208):375-400. doi: 10.1007/978-3-642-23274-9_16. Handb Exp Pharmacol. 2012. PMID: 22222707 Review.
-
Muscarinic receptor agonists and antagonists: effects on keratinocyte functions.Handb Exp Pharmacol. 2012;(208):429-50. doi: 10.1007/978-3-642-23274-9_18. Handb Exp Pharmacol. 2012. PMID: 22222709 Review.
Cited by
-
Targeting Perineural Invasion in Pancreatic Cancer.Cancers (Basel). 2024 Dec 21;16(24):4260. doi: 10.3390/cancers16244260. Cancers (Basel). 2024. PMID: 39766161 Free PMC article. Review.
-
The protective role of anti-parkinsonian drugs in pancreatic cancer risk: A comprehensive case-control study in Taiwan.Cancer Sci. 2025 Mar;116(3):783-791. doi: 10.1111/cas.16422. Epub 2024 Dec 4. Cancer Sci. 2025. PMID: 39629516 Free PMC article.
-
Acetylcholine signaling system in progression of lung cancers.Pharmacol Ther. 2019 Feb;194:222-254. doi: 10.1016/j.pharmthera.2018.10.002. Epub 2018 Oct 3. Pharmacol Ther. 2019. PMID: 30291908 Free PMC article. Review.
-
Aclidinium inhibits proliferation and metastasis of ovarian cancer SKOV3 cells via downregulating PI3K/AKT/mTOR signaling pathway.Oncol Lett. 2018 Nov;16(5):6417-6422. doi: 10.3892/ol.2018.9460. Epub 2018 Sep 19. Oncol Lett. 2018. PMID: 30405778 Free PMC article.
-
Delirium from the gliocentric perspective.Front Cell Neurosci. 2015 May 11;9:171. doi: 10.3389/fncel.2015.00171. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26029046 Free PMC article.
References
-
- Ackerman MS, Roeske WR, Heck RJ, Korc M. Identification and characterization of muscarinic receptors in cultured human pancreatic carcinoma cells. Pancreas. 1989;4:363–370. - PubMed
-
- Aihara T, Nakamura Y, Taketo MM, Matsui M, Okabe S. Cholinergically stimulated gastric acid secretion is mediated by M(3) and M(5) but not M(1) muscarinic acetylcholine receptors in mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1199–G1207. - PubMed
-
- Arredondo J, Hall LL, Ndoye A, Chernyavsky AI, Jolkovsky DL, Grando SA. Muscarinic acetylcholine receptors regulating cell cycle progression are expressed in human gingival keratinocytes. J Periodontal Res. 2003;38:79–89. - PubMed
-
- Ashkenazi A, Ramachandran J, Capon DJ. Acetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypes. Nature. 1989;340:146–150. - PubMed
-
- Batra S, Popper LD, Iosif CS. Characterisation of muscarinic cholinergic receptors in human ovaries, ovarian tumours and tumour cell lines. Eur J Cancer. 1993;29A:1302–1306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

