Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing α4 and α6 subunits
- PMID: 22222765
- PMCID: PMC3310415
- DOI: 10.1124/mol.111.076661
Nicotine persistently activates ventral tegmental area dopaminergic neurons via nicotinic acetylcholine receptors containing α4 and α6 subunits
Abstract
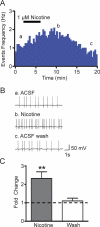
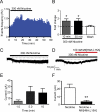
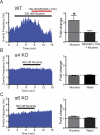
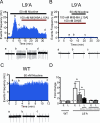
Nicotine is reinforcing because it activates dopaminergic (DAergic) neurons within the ventral tegmental area (VTA) of the brain's mesocorticolimbic reward circuitry. This increase in activity can occur for a period of several minutes up to an hour and is thought to be a critical component of nicotine dependence. However, nicotine concentrations that are routinely self-administered by smokers are predicted to desensitize high-affinity α4β2 neuronal nicotinic acetylcholine receptors (nAChRs) in seconds. Thus, how physiologically relevant nicotine concentrations persistently activate VTA DAergic neurons is unknown. Here we show that nicotine can directly and robustly increase the firing frequency of VTA DAergic neurons for several minutes. In mouse midbrain slices, 300 nM nicotine elicited a persistent inward current in VTA DAergic neurons that was blocked by α-conotoxin MII[H9A;L15A], a selective antagonist of nAChRs containing the α6 subunit. α-conotoxin MII[H9A;L15A] also significantly reduced the long-lasting increase in DAergic neuronal activity produced by low concentrations of nicotine. In addition, nicotine failed to significantly activate VTA DAergic neurons in mice that did not express either α4 or α6 nAChR subunits. Conversely, selective activation of nAChRs containing the α4 subunit in knock-in mice expressing a hypersensitive version of these receptors yielded a biphasic response to nicotine consisting of an acute desensitizing increase in firing frequency followed by a sustained increase that lasted several minutes and was sensitive to α-conotoxin MII[H9A;L15A]. These data indicate that nicotine persistently activates VTA DAergic neurons via nAChRs containing α4 and α6 subunits.
Figures

Similar articles
-
Nicotinic acetylcholine receptors containing the α6 subunit contribute to ethanol activation of ventral tegmental area dopaminergic neurons.Biochem Pharmacol. 2013 Oct 15;86(8):1194-200. doi: 10.1016/j.bcp.2013.06.015. Epub 2013 Jun 26. Biochem Pharmacol. 2013. PMID: 23811312 Free PMC article.
-
Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area.Neuropsychopharmacology. 2011 Apr;36(5):1021-32. doi: 10.1038/npp.2010.240. Epub 2011 Feb 2. Neuropsychopharmacology. 2011. PMID: 21289604 Free PMC article.
-
Functional nicotinic acetylcholine receptors containing α6 subunits are on GABAergic neuronal boutons adherent to ventral tegmental area dopamine neurons.J Neurosci. 2011 Feb 16;31(7):2537-48. doi: 10.1523/JNEUROSCI.3003-10.2011. J Neurosci. 2011. PMID: 21325521 Free PMC article.
-
Mysterious alpha6-containing nAChRs: function, pharmacology, and pathophysiology.Acta Pharmacol Sin. 2009 Jun;30(6):740-51. doi: 10.1038/aps.2009.63. Acta Pharmacol Sin. 2009. PMID: 19498417 Free PMC article. Review.
-
Cortical control of VTA function and influence on nicotine reward.Biochem Pharmacol. 2013 Oct 15;86(8):1173-80. doi: 10.1016/j.bcp.2013.07.013. Epub 2013 Aug 7. Biochem Pharmacol. 2013. PMID: 23933294 Review.
Cited by
-
Positional scanning mutagenesis of α-conotoxin PeIA identifies critical residues that confer potency and selectivity for α6/α3β2β3 and α3β2 nicotinic acetylcholine receptors.J Biol Chem. 2013 Aug 30;288(35):25428-25439. doi: 10.1074/jbc.M113.482059. Epub 2013 Jul 11. J Biol Chem. 2013. PMID: 23846688 Free PMC article.
-
Modulation of ethanol reward sensitivity by nicotinic acetylcholine receptors containing the α6 subunit.Alcohol. 2016 Dec;57:65-70. doi: 10.1016/j.alcohol.2016.08.006. Epub 2016 Oct 8. Alcohol. 2016. PMID: 27793544 Free PMC article.
-
Why flavored vape products may be attractive: Green apple tobacco flavor elicits reward-related behavior, upregulates nAChRs on VTA dopamine neurons, and alters midbrain dopamine and GABA neuron function.Neuropharmacology. 2019 Nov 1;158:107729. doi: 10.1016/j.neuropharm.2019.107729. Epub 2019 Jul 29. Neuropharmacology. 2019. PMID: 31369741 Free PMC article.
-
α-Conotoxin PeIA[S9H,V10A,E14N] potently and selectively blocks α6β2β3 versus α6β4 nicotinic acetylcholine receptors.Mol Pharmacol. 2012 Nov;82(5):972-82. doi: 10.1124/mol.112.080853. Epub 2012 Aug 22. Mol Pharmacol. 2012. PMID: 22914547 Free PMC article.
-
Ventral tegmental area α6β2 nicotinic acetylcholine receptors modulate phasic dopamine release in the nucleus accumbens core.Psychopharmacology (Berl). 2013 Sep;229(1):73-82. doi: 10.1007/s00213-013-3082-0. Epub 2013 Apr 30. Psychopharmacology (Berl). 2013. PMID: 23624852 Free PMC article.
References
-
- Brodie MS. (1991) Low concentrations of nicotine increase the firing rate of neurons of the rat ventral tegmental area in vitro. Birkhauser Verlag; Basel
-
- Centers for Disease Control and Prevention (2010) Vital signs: current cigarette smoking among adults aged > or =18 years—United States, 2009. MMWR Morb Mortal Wkly Rep 59:1135–1140 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

