Quantitative monitoring of mouse lung tumors by magnetic resonance imaging
- PMID: 22222788
- PMCID: PMC3870466
- DOI: 10.1038/nprot.2011.424
Quantitative monitoring of mouse lung tumors by magnetic resonance imaging
Abstract
Primary lung cancer remains the leading cause of cancer-related death in the Western world, and the lung is a common site for recurrence of extrathoracic malignancies. Small-animal (rodent) models of cancer can have a very valuable role in the development of improved therapeutic strategies. However, detection of mouse pulmonary tumors and their subsequent response to therapy in situ is challenging. We have recently described MRI as a reliable, reproducible and nondestructive modality for the detection and serial monitoring of pulmonary tumors. By combining respiratory-gated data acquisition methods with manual and automated segmentation algorithms described by our laboratory, pulmonary tumor burden can be quantitatively measured in approximately 1 h (data acquisition plus analysis) per mouse. Quantitative, analytical methods are described for measuring tumor burden in both primary (discrete tumors) and metastatic (diffuse tumors) disease. Thus, small-animal MRI represents a novel and unique research tool for preclinical investigation of therapeutic strategies for treatment of pulmonary malignancies, and it may be valuable in evaluating new compounds targeting lung cancer in vivo.
Figures



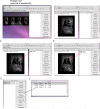
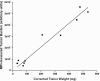
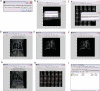
Similar articles
-
Quantitative analysis of tumor burden in mouse lung via MRI.Magn Reson Med. 2012 Feb;67(2):572-9. doi: 10.1002/mrm.22951. Epub 2011 Sep 27. Magn Reson Med. 2012. PMID: 21954021 Free PMC article.
-
Detection of primary lung tumors in rodents by magnetic resonance imaging.Cancer Res. 2004 Apr 15;64(8):2740-2. doi: 10.1158/0008-5472.can-03-3258. Cancer Res. 2004. PMID: 15087388
-
Quantitative monitoring of adenocarcinoma development in rodents by magnetic resonance imaging.Clin Cancer Res. 2008 Mar 1;14(5):1363-7. doi: 10.1158/1078-0432.CCR-07-1757. Clin Cancer Res. 2008. PMID: 18316556
-
Imaging lung cancer.Semin Oncol. 1999 Oct;26(5 Suppl 15):21-6. Semin Oncol. 1999. PMID: 10566607 Review.
-
MR imaging of pulmonary and mediastinal malignancies.Magn Reson Imaging Clin N Am. 2000 Nov;8(4):729-39. Magn Reson Imaging Clin N Am. 2000. PMID: 11149676 Review.
Cited by
-
Automated computer quantification of breast cancer in small-animal models using PET-guided MR image co-segmentation.EJNMMI Res. 2013 Jul 5;3(1):49. doi: 10.1186/2191-219X-3-49. EJNMMI Res. 2013. PMID: 23829944 Free PMC article.
-
Cardio-Respiratory synchronized bSSFP MRI for high throughput in vivo lung tumour quantification.PLoS One. 2019 Feb 12;14(2):e0212172. doi: 10.1371/journal.pone.0212172. eCollection 2019. PLoS One. 2019. PMID: 30753240 Free PMC article.
-
NRF2 Activation Promotes Aggressive Lung Cancer and Associates with Poor Clinical Outcomes.Clin Cancer Res. 2021 Feb 1;27(3):877-888. doi: 10.1158/1078-0432.CCR-20-1985. Epub 2020 Oct 19. Clin Cancer Res. 2021. PMID: 33077574 Free PMC article.
-
Imaging preclinical tumour models: improving translational power.Nat Rev Cancer. 2014 Jul;14(7):481-93. doi: 10.1038/nrc3751. Epub 2014 Jun 19. Nat Rev Cancer. 2014. PMID: 24943811 Review.
-
Biomarkers in preclinical cancer imaging.Eur J Nucl Med Mol Imaging. 2015 Apr;42(4):579-96. doi: 10.1007/s00259-014-2980-7. Epub 2015 Feb 12. Eur J Nucl Med Mol Imaging. 2015. PMID: 25673052 Free PMC article. Review.
References
-
- Ghetie C, Davies M, Cornfeld D, Suh N, Saif MW. Expectoration of a lung metastasis in a patient with colorectal carcinoma. Clin Colorectal Cancer. 2008;7:283–286. - PubMed
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Leong SP, et al. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006;25:221–232. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

