Hypoxia sensitization of hepatocytes to neutrophil elastase-mediated cell death depends on MAPKs and HIF-1α
- PMID: 22223132
- PMCID: PMC3330781
- DOI: 10.1152/ajpgi.00409.2011
Hypoxia sensitization of hepatocytes to neutrophil elastase-mediated cell death depends on MAPKs and HIF-1α
Abstract
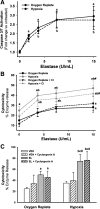
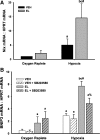
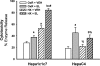
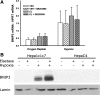
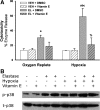
The liver is sensitive to pathological conditions associated with tissue hypoxia (Hx) and the presence of activated neutrophils that secrete the serine protease elastase (EL). We demonstrated previously that cotreatment of rat hepatocytes with nontoxic levels of Hx and EL caused synergistic cell death. Hx is sensed by hypoxia-inducible factor (HIF)-1α, a transcription factor that heterodimerizes with HIF-1β/aryl hydrocarbon receptor nuclear translocator and directs expression of many genes, including the pro-cell death gene Bcl-2/adenovirus E1B-interacting protein 3 (BNIP3). Since cell death from EL or Hx also requires MAPK activation, we tested the hypothesis that the cytotoxic interaction of Hx and EL depends on MAPK and HIF-1α signaling. Treatment of Hepa1c1c7 cells with EL in the presence of Hx (2% O(2)) resulted in synergistic cell death. EL reduced phosphorylated ERK in O(2)-replete and Hx-exposed cells, and ERK inhibition enhanced the cytotoxicity of EL alone. Hx-EL cotreatment caused an additive increase in phosphorylated p38, and p38 inhibition attenuated cell death caused by this cotreatment. EL enhanced Hx-induced HIF-1α accumulation and transcription of the HIF-1α-mediated cell death gene BNIP3, and p38 inhibition attenuated BNIP3 expression and production. Cytotoxicity and BNIP3 expression from EL-Hx cotreatment were reduced in HIF-1β-deficient HepaC4 cells compared with Hepa1c1c7 cells. These results suggest that p38 signaling contributes to Hx-EL cotreatment-induced cell death via modulation of HIF-1α-mediated gene transcription. Finally, lipid peroxidation was enhanced in Hx-EL-cotreated cells compared with cells treated with EL or Hx alone. Vitamin E treatment attenuated lipid peroxidation and protected cells from the cytotoxicity of Hx and EL, suggesting that lipid peroxidation plays a role.
Figures






References
-
- Arteel GE, Iimuro Y, Yin M, Raleigh JA, Thurman RG. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology 25: 920–926, 1997 - PubMed
-
- Choi SM, Oh H, Park H. Microarray analyses of hypoxia-regulated genes in an aryl hydrocarbon receptor nuclear translocator (Arnt)-dependent manner. FEBS J 275: 5618–5634, 2008 - PubMed
-
- Chu W, Li X, Li C, Wan L, Shi H, Song X, Liu X, Chen X, Zhang C, Shan H, Lu Y, Yang B. TGFBR3, a potential negative regulator of TGF-β signaling, protects cardiac fibroblasts from hypoxia-induced apoptosis. J Cell Physiol 226: 2586–2594, 2011 - PubMed
-
- Conrad PW, Rust RT, Han J, Millhorn DE, Beitner-Johnson D. Selective activation of p38α and p38γ by hypoxia. Role in regulation of cyclin D1 by hypoxia in PC12 cells. J Biol Chem 274: 23570–23576, 1999 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

