Enzymatic total synthesis of defucogilvocarcin M and its implications for gilvocarcin biosynthesis
- PMID: 22223167
- PMCID: PMC3343456
- DOI: 10.1002/anie.201105882
Enzymatic total synthesis of defucogilvocarcin M and its implications for gilvocarcin biosynthesis
Figures



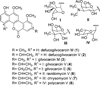

References
-
- Fischer C, Lipata F, Rohr J. J. Am. Chem. Soc. 2003;125:7818–7819. - PMC - PubMed
- Takahashi K, Yoshida M, Tomita F, Shirahata K. J. Antibiot. 1981;34:271–275. - PubMed
- Hatano K, Hagashide E, Shibata M, Kameda Y, Horii S. Agric. Biol. Chem. 1980;44:1157–1163.
- Balitz DM, O’Herron FA, Bush J, Vyas DM, Nettleton DE, Grulich RE, Bradner WT, Doyle TW, Arnold E, Clardy J. J. Antibiot. 1981;34:1544–1555. - PubMed
-
- Sehgal SN, Czerkawski H, Kudelski A, Pandev K, Saucier R, Vezina C. J. Antibiot. 1983;36:355–361. - PubMed
- Weiss U, Yoshihira K, Highet RJ, White RJ, Wei TT. J. Antibiot. 1982;35:1194–1201. - PubMed
- Li YQ, Huang XS, Ishida K, Maier A, Kelter G, Jiang Y, Peschel G, Menzel KD, Li MG, Wen ML, Xu LH, Grabley S, Fiebig HH, Jiang CL, Hertweck C, Sattler I. Org. Biomol. Chem. 2008;6:3601–3605. - PubMed
- Nakajima S, Kojiri K, Suda H, Okanishi M. J. Antibiot. 1991;44:1061–1064. - PubMed
- Yamashita N, Shin-ya K, Furihata K, Hayakawa Y, Seto H. J. Antibiot. 1998;51:1105–1108. - PubMed
-
- Matsumoto A, Hanawalt PC. Cancer Res. 2000;60:3921–3926. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

