Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death
- PMID: 22223353
- PMCID: PMC4068617
- DOI: 10.1161/CIRCRESAHA.111.260455
Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death
Abstract
Rationale: Diastolic spontaneous Ca(2+) waves (DCWs) are recognized as important contributors to triggered arrhythmias. DCWs are thought to arise when [Ca(2+)] in sarcoplasmic reticulum ([Ca(2+)](SR)) reaches a certain threshold level, which might be reduced in cardiac disease as a consequence of sensitization of ryanodine receptors (RyR2s) to luminal Ca(2+).
Objective: We investigated the mechanisms of DCW generation in myocytes from normal and diseased hearts, using a canine model of post-myocardial infarction ventricular fibrillation (VF).
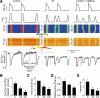
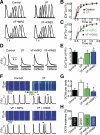
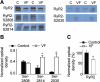
Methods and results: The frequency of DCWs, recorded during periodic pacing in the presence of a β-adrenergic receptor agonist isoproterenol, was significantly higher in VF myocytes than in normal controls. Rather than occurring immediately on reaching a final [Ca(2+)](SR), DCWs arose with a distinct time delay after attaining steady [Ca(2+)](SR) in both experimental groups. Although the rate of [Ca(2+)](SR) recovery after the SR Ca(2+) release was similar between the groups, in VF myocytes the latency to DCWs was shorter, and the [Ca(2+)](SR) at DCW initiation was lower. The restitution of depolarization-induced Ca(2+) transients, assessed by a 2-pulse protocol, was significantly faster in VF myocytes than in controls. The VF-related alterations in myocyte Ca(2+) cycling were mimicked by the RyR2 agonist, caffeine. The reducing agent, mercaptopropionylglycine, or the CaMKII inhibitor, KN93, decreased DCW frequency and normalized restitution of Ca(2+) release in VF myocytes.
Conclusions: The attainment of a certain threshold [Ca(2+)](SR) is not sufficient for the generation of DCWs. Postrelease Ca(2+) signaling refractoriness critically influences the occurrence of DCWs. Shortened Ca(2+) signaling refractoriness due to RyR2 phosphorylation and oxidation is responsible for the increased rate of DCWs observed in VF myocytes and could provide a substrate for synchronization of arrhythmogenic events at the tissue level in hearts prone to VF.
Figures



References
-
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics: 2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. - PubMed
-
- Bunch TJ, Hohnloser SH, Gersh BJ. Mechanisms of sudden cardiac death in myocardial infarction survivors: insights from the randomized trials of implantable cardioverter-defibrillators. Circulation. 2007;115:2451–2457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

