Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices
- PMID: 22223464
- PMCID: PMC3276747
- DOI: 10.1002/glia.22287
Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices
Abstract
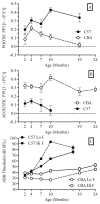
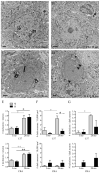
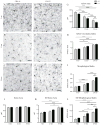
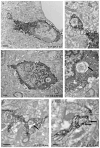
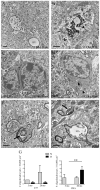
Normal aging is often accompanied by a progressive loss of receptor sensitivity in hearing and vision, whose consequences on cellular function in cortical sensory areas have remained largely unknown. By examining the primary auditory (A1) and visual (V1) cortices in two inbred strains of mice undergoing either age-related loss of audition (C57BL/6J) or vision (CBA/CaJ), we were able to describe cellular and subcellular changes that were associated with normal aging (occurring in A1 and V1 of both strains) or specifically with age-related sensory loss (only in A1 of C57BL/6J or V1 of CBA/CaJ), using immunocytochemical electron microscopy and light microscopy. While the changes were subtle in neurons, glial cells and especially microglia were transformed in aged animals. Microglia became more numerous and irregularly distributed, displayed more variable cell body and process morphologies, occupied smaller territories, and accumulated phagocytic inclusions that often displayed ultrastructural features of synaptic elements. Additionally, evidence of myelination defects were observed, and aged oligodendrocytes became more numerous and were more often encountered in contiguous pairs. Most of these effects were profoundly exacerbated by age-related sensory loss. Together, our results suggest that the age-related alteration of glial cells in sensory cortical areas can be accelerated by activity-driven central mechanisms that result from an age-related loss of peripheral sensitivity. In light of our observations, these age-related changes in sensory function should be considered when investigating cellular, cortical, and behavioral functions throughout the lifespan in these commonly used C57BL/6J and CBA/CaJ mouse models.
Copyright © 2012 Wiley Periodicals, Inc.
Figures



References
-
- Anderson JM, Hubbard BM, Coghill GR, Slidders W. The effect of advanced old age on the neurone content of the cerebral cortex. Observations with an automatic image analyser point counting method. J Neurol Sci. 1983;58:235–46. - PubMed
-
- Auer RN, Kalimo H, Olsson Y, Siesjo BK. The temporal evolution of hypoglycemic brain damage. I. Light- and electron-microscopic findings in the rat cerebral cortex. Acta Neuropathol. 1985;67:13–24. - PubMed
-
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96:5280–5. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

