Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins
- PMID: 22223710
- PMCID: PMC3271813
- DOI: 10.1093/aje/kwr301
Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins
Abstract
Randomized clinical trials (RCTs) are usually the preferred strategy with which to generate evidence of comparative effectiveness, but conducting an RCT is not always feasible. Though observational studies and RCTs often provide comparable estimates, the questioning of observational analyses has recently intensified because of randomized-observational discrepancies regarding the effect of postmenopausal hormone replacement therapy on coronary heart disease. Reanalyses of observational data that excluded prevalent users of hormone replacement therapy led to attenuated discrepancies, which begs the question of whether exclusion of prevalent users should be generally recommended. In the current study, the authors evaluated the effect of excluding prevalent users of statins in a meta-analysis of observational studies of persons with cardiovascular disease. The pooled, multivariate-adjusted mortality hazard ratio for statin use was 0.77 (95% confidence interval (CI): 0.65, 0.91) in 4 studies that compared incident users with nonusers, 0.70 (95% CI: 0.64, 0.78) in 13 studies that compared a combination of prevalent and incident users with nonusers, and 0.54 (95% CI: 0.45, 0.66) in 13 studies that compared prevalent users with nonusers. The corresponding hazard ratio from 18 RCTs was 0.84 (95% CI: 0.77, 0.91). It appears that the greater the proportion of prevalent statin users in observational studies, the larger the discrepancy between observational and randomized estimates.
Figures
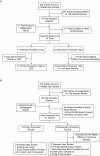
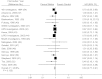
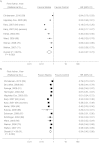


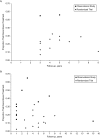
References
-
- Avorn J. Debate about funding comparative-effectiveness research. N Engl J Med. 2009;360(19):1927–1929. - PubMed
-
- Iglehart JK. Prioritizing comparative-effectiveness research—IOM recommendations. N Engl J Med. 2009;361(4):325–328. - PubMed
-
- Kuehn BM. Institute of Medicine outlines priorities for comparative effectiveness research. JAMA. 2009;302(9):936–937. - PubMed
-
- Mushlin AI, Ghomrawi H. Health care reform and the need for comparative-effectiveness research. N Engl J Med. 2010;362(3):e6. (doi:10.1056/NEJMp0912651) - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

