Negative effects of GM-CSF signaling in a murine model of t(8;21)-induced leukemia
- PMID: 22223820
- PMCID: PMC3321875
- DOI: 10.1182/blood-2011-04-350694
Negative effects of GM-CSF signaling in a murine model of t(8;21)-induced leukemia
Abstract
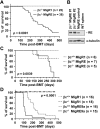
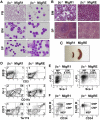
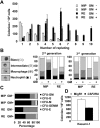
The t(8;21)(q22;q22) is common in adult acute myeloid leukemia (AML). The RUNX1-ETO fusion protein that is expressed by this translocation is poorly leukemogenic and requires additional mutations for transformation. Loss of sex chromosome (LOS) is frequently observed in t(8;21) AML. In the present study, to evaluate whether LOS cooperates with t(8;21) in leukemogenesis, we first used a retroviral transduction/transplantation model to express RUNX1-ETO in hematopoietic cells from XO mice. The low frequency of leukemia in these mice suggests that the potentially critical gene for suppression of t(8;21) leukemia in humans is not conserved on mouse sex chromosomes. The gene encoding the GM-CSF receptor α subunit (CSF2RA) is located on X and Y chromosomes in humans but on chromosome 19 in mice. GM-CSF promotes myeloid cell survival, proliferation, and differentiation. To determine whether GM-CSF signaling affects RUNX1-ETO leukemogenesis, hematopoietic stem/progenitor cells that lack GM-CSF signaling were used to express RUNX1-ETO and transplanted into lethally irradiated mice, and a high penetrance of AML was observed in recipients. Furthermore, GM-CSF reduced the replating ability of RUNX1-ETO-expressing cells. These results suggest a possible tumor-suppressor role of GM-CSF in RUNX1-ETO leukemia. Loss of the CSF2RA gene may be a critical mutation explaining the high incidence of LOS associated with the t(8;21)(q22;q22) translocation.
Figures

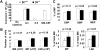
Comment in
-
GM-CSFRα: the sex-chromosome link to t(8;21)(+) AML?Blood. 2012 Mar 29;119(13):2976-7. doi: 10.1182/blood-2012-01-403691. Blood. 2012. PMID: 22461472
Similar articles
-
Restoration of MYC-repressed targets mediates the negative effects of GM-CSF on RUNX1-ETO leukemogenicity.Leukemia. 2017 Jan;31(1):159-169. doi: 10.1038/leu.2016.167. Epub 2016 Jun 15. Leukemia. 2017. PMID: 27389055 Free PMC article.
-
The AML1-ETO fusion gene and the FLT3 length mutation collaborate in inducing acute leukemia in mice.J Clin Invest. 2005 Aug;115(8):2159-68. doi: 10.1172/JCI24225. Epub 2005 Jul 14. J Clin Invest. 2005. PMID: 16025155 Free PMC article.
-
Persistence of multipotent progenitors expressing AML1/ETO transcripts in long-term remission patients with t(8;21) acute myelogenous leukemia.Blood. 1996 Jun 1;87(11):4789-96. Blood. 1996. PMID: 8639850
-
Acute myeloid leukemia with the 8q22;21q22 translocation: secondary mutational events and alternative t(8;21) transcripts.Blood. 2007 Aug 1;110(3):799-805. doi: 10.1182/blood-2006-11-019265. Epub 2007 Apr 5. Blood. 2007. PMID: 17412887 Free PMC article. Review.
-
Molecular targeting of aberrant transcription factors in leukemia: strategies for RUNX1/ETO.Curr Drug Targets. 2010 Sep;11(9):1181-91. doi: 10.2174/138945010792006744. Curr Drug Targets. 2010. PMID: 20583973 Review.
Cited by
-
Sex chromosome loss and the pseudoautosomal region genes in hematological malignancies.Oncotarget. 2016 Nov 1;7(44):72356-72372. doi: 10.18632/oncotarget.12050. Oncotarget. 2016. PMID: 27655702 Free PMC article. Review.
-
Monocytes reprogrammed with lentiviral vectors co-expressing GM-CSF, IFN-α2 and antigens for personalized immune therapy of acute leukemia pre- or post-stem cell transplantation.Cancer Immunol Immunother. 2019 Nov;68(11):1891-1899. doi: 10.1007/s00262-019-02406-9. Epub 2019 Oct 18. Cancer Immunol Immunother. 2019. PMID: 31628525 Free PMC article. Review.
-
Core Binding Factor Leukemia: Chromatin Remodeling Moves Towards Oncogenic Transcription.Cancers (Basel). 2019 Dec 7;11(12):1973. doi: 10.3390/cancers11121973. Cancers (Basel). 2019. PMID: 31817911 Free PMC article. Review.
-
Deficiency of β Common Receptor Moderately Attenuates the Progression of Myeloproliferative Neoplasm in NrasG12D/+ Mice.J Biol Chem. 2015 Jul 31;290(31):19093-103. doi: 10.1074/jbc.M115.653154. Epub 2015 Jun 16. J Biol Chem. 2015. PMID: 26082490 Free PMC article.
-
RUNX1-ETO: Attacking the Epigenome for Genomic Instable Leukemia.Int J Mol Sci. 2019 Jan 16;20(2):350. doi: 10.3390/ijms20020350. Int J Mol Sci. 2019. PMID: 30654457 Free PMC article. Review.
References
-
- Yergeau DA, Hetherington CJ, Wang Q, et al. Embryonic lethality and impairment of haematopoiesis in mice heterozygous for an AML1-ETO fusion gene. Nat Genet. 1997;15(3):303–306. - PubMed
-
- Okuda T, Cai Z, Yang S, et al. Expression of a knocked-in AML1-ETO leukemia gene inhibits the establishment of normal definitive hematopoiesis and directly generates dysplastic hematopoietic progenitors. Blood. 1998;91(9):3134–3143. - PubMed
-
- Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84(2):321–330. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

