Protective antigen antibody augments hemodynamic support in anthrax lethal toxin shock in canines
- PMID: 22223857
- PMCID: PMC3274375
- DOI: 10.1093/infdis/jir834
Protective antigen antibody augments hemodynamic support in anthrax lethal toxin shock in canines
Abstract
Background: Anthrax-associated shock is closely linked to lethal toxin (LT) release and is highly lethal despite conventional hemodynamic support. We investigated whether protective antigen-directed monoclonal antibody (PA-mAb) treatment further augments titrated hemodynamic support.
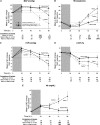
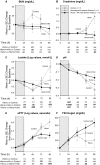
Methods and results: Forty sedated, mechanically ventilated, instrumented canines challenged with anthrax LT were assigned to no treatment (controls), hemodynamic support alone (protocol-titrated fluids and norepinephrine), PA-mAb alone (administered at start of LT infusion [0 hours] or 9 or 12 hours later), or both, and observed for 96 hours. Although all 8 controls died, 2 of 8 animals receiving hemodynamic support alone survived (median survival times 65 vs 85 hours, respectively; P = .03). PA-mAb alone at 0 hour improved survival (5 of 5 animals survived), but efficacy decreased progressively with delayed treatment (9 hours, 2 of 3 survived; 12 hours, 0 of 4 survived) (P = .004 comparing survival across treatment times). However, combined treatment increased survival irrespective of PA-mAb administration time (0 hours, 4 of 5 animals; 9 hours, 3 of 3 animals; and 12 hours, 4 of 5 animals survived) (P = .95 comparing treatment times). Compared to hemodynamic support alone, when combined over PA-mAb treatment times (0, 9, and 12 hours), combination therapy produced higher survival (P = .008), central venous pressures, and left ventricular ejection fractions, and lower heart rates, norepinephrine requirements and fluid retention (P ≤ .03).
Conclusions: PA-mAb may augment conventional hemodynamic support during anthrax LT-associated shock.
Figures





Similar articles
-
A Review of the Efficacy of FDA-Approved B. anthracis Anti-Toxin Agents When Combined with Antibiotic or Hemodynamic Support in Infection- or Toxin-Challenged Preclinical Models.Toxins (Basel). 2021 Jan 13;13(1):53. doi: 10.3390/toxins13010053. Toxins (Basel). 2021. PMID: 33450877 Free PMC article. Review.
-
Late treatment with a protective antigen-directed monoclonal antibody improves hemodynamic function and survival in a lethal toxin-infused rat model of anthrax sepsis.J Infect Dis. 2005 Feb 1;191(3):422-34. doi: 10.1086/427189. Epub 2004 Dec 22. J Infect Dis. 2005. PMID: 15633102
-
Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax.Infect Immun. 2006 Feb;74(2):1016-24. doi: 10.1128/IAI.74.2.1016-1024.2006. Infect Immun. 2006. PMID: 16428748 Free PMC article.
-
Human monoclonal anti-protective antigen antibody for the low-dose post-exposure prophylaxis and treatment of Anthrax.BMC Infect Dis. 2018 Dec 10;18(1):640. doi: 10.1186/s12879-018-3542-6. BMC Infect Dis. 2018. PMID: 30526504 Free PMC article.
-
Monoclonal antibody therapies against anthrax.Toxins (Basel). 2011 Aug;3(8):1004-19. doi: 10.3390/toxins3081004. Epub 2011 Aug 15. Toxins (Basel). 2011. PMID: 22069754 Free PMC article. Review.
Cited by
-
An overview of anthrax infection including the recently identified form of disease in injection drug users.Intensive Care Med. 2012 Jul;38(7):1092-104. doi: 10.1007/s00134-012-2541-0. Epub 2012 Apr 24. Intensive Care Med. 2012. PMID: 22527064 Free PMC article. Review.
-
Bacillus anthracis edema but not lethal toxin challenge in rats is associated with depressed myocardial function in hearts isolated and tested in a Langendorff system.Am J Physiol Heart Circ Physiol. 2015 Jun 15;308(12):H1592-602. doi: 10.1152/ajpheart.00851.2014. Epub 2015 Apr 10. Am J Physiol Heart Circ Physiol. 2015. PMID: 25862834 Free PMC article.
-
A Review of the Efficacy of FDA-Approved B. anthracis Anti-Toxin Agents When Combined with Antibiotic or Hemodynamic Support in Infection- or Toxin-Challenged Preclinical Models.Toxins (Basel). 2021 Jan 13;13(1):53. doi: 10.3390/toxins13010053. Toxins (Basel). 2021. PMID: 33450877 Free PMC article. Review.
-
A systematic review and meta-analysis of preclinical trials testing anti-toxin therapies for B. anthracis infection: A need for more robust study designs and results.PLoS One. 2017 Aug 10;12(8):e0182879. doi: 10.1371/journal.pone.0182879. eCollection 2017. PLoS One. 2017. PMID: 28797061 Free PMC article.
-
B. anthracis edema toxin increases cAMP levels and inhibits phenylephrine-stimulated contraction in a rat aortic ring model.Am J Physiol Heart Circ Physiol. 2013 Jul 15;305(2):H238-50. doi: 10.1152/ajpheart.00185.2013. Epub 2013 Apr 12. Am J Physiol Heart Circ Physiol. 2013. PMID: 23585140 Free PMC article.
References
-
- Centers for Disease Control and Prevention (CDC) Update: investigation of bioterrorism-related inhalational anthrax–Connecticut, 2001. MMWR Morb Mortal Wkly Rep. 2001;50:1049–51. - PubMed
-
- Booth MG, Hood J, Brooks TJ, Hart A. Anthrax infection in drug users. Lancet. 2010;375:1345–6. - PubMed
-
- Anthrax outbreak among heroin users in the United Kingdom and Germany. http://www.emcdda.europa.eu/online/annual-report/2010/boxes/p83. Accessed 1 June 2011.
-
- Collier RJ, Young JA. Anthrax toxin. Annu Rev Cell Dev Biol. 2003;19:45–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

