Multiple factors influence glomerular albumin permeability in rats
- PMID: 22223875
- PMCID: PMC3294301
- DOI: 10.1681/ASN.2011070666
Multiple factors influence glomerular albumin permeability in rats
Abstract
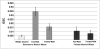
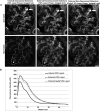
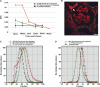
Different laboratories recently reported incongruous results describing the quantification of albumin filtration using two-photon microscopy. We investigated the factors that influence the glomerular sieving coefficient for albumin (GSC(A)) in an effort to explain these discordant reports and to develop standard operating procedures for determining GSC(A). Multiple factors influenced GSC(A), including the kidney depth of image acquisition (10-20 μm was appropriate), the selection of fluorophore (probes emitting longer wavelengths were superior), the selection of plasma regions for fluorescence measurements, the size and molecular dispersion characteristics of dextran polymers if used, dietary status, and the genetic strain of rat. Fasting reduced the GSC(A) in Simonsen Munich Wistar rats from 0.035±0.005 to 0.016±0.004 (P<0.01). Frömter Munich Wistar rats had a much lower GSC(A) in both the fed and the fasted states. Finally, we documented extensive albumin transcytosis with vesicular and tubular delivery to and fusion with the basolateral membrane in S1 proximal tubule cells. In summary, these results help explain the previously conflicting microscopy and micropuncture data describing albumin filtration and highlight the dynamic nature of glomerular albumin permeability.
Figures




Comment in
-
Illuminating the glomerular filtration barrier, two photons at a time.J Am Soc Nephrol. 2012 Mar;23(3):373-5. doi: 10.1681/ASN.2012010067. Epub 2012 Feb 9. J Am Soc Nephrol. 2012. PMID: 22323639 No abstract available.
References
-
- Haraldsson B, Nyström J, Deen WM: Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 88: 451–487, 2008 - PubMed
-
- Remuzzi A, Sangalli F, Fassi A, Remuzzi G: Albumin concentration in the Bowman’s capsule: Multiphoton microscopy vs micropuncture technique. Kidney Int 72: 1410–1411, author reply 1411, 2007 - PubMed
-
- Wang Y, Cai H, Cebotaru L, Hryciw DH, Weinman EJ, Donowitz M, Guggino SE, Guggino WB: ClC-5: Role in endocytosis in the proximal tubule. Am J Physiol Renal Physiol 289: F850–F862, 2005 - PubMed
-
- Rangel-Filho A, Sharma M, Datta YH, Moreno C, Roman RJ, Iwamoto Y, Provoost AP, Lazar J, Jacob HJ: RF-2 gene modulates proteinuria and albuminuria independently of changes in glomerular permeability in the fawn-hooded hypertensive rat. J Am Soc Nephrol 16: 852–856, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

