Adenosine-5'-triphosphate up-regulates proliferation of human cardiac fibroblasts
- PMID: 22224416
- PMCID: PMC3417435
- DOI: 10.1111/j.1476-5381.2012.01831.x
Adenosine-5'-triphosphate up-regulates proliferation of human cardiac fibroblasts
Abstract
Background and purpose: ATP is a potent signalling molecule that regulates biological activities including increasing or decreasing proliferation in different types of cells. The aim of the present study was to investigate how ATP regulates the proliferation of human cardiac fibroblasts.
Experimental approach: Reverse transcription (RT)-PCR, Western blot analysis, cell proliferation and migration assays were employed to investigate the effects of ATP on human adult ventricular fibroblasts.
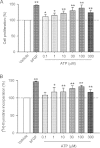
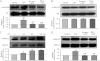
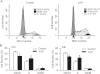
Key results: ATP increased cell proliferation in a concentration-dependent manner. Similarly, the P2X receptor agonist α,β-methylene ATP and P2Y receptor agonist ATP-γS also up-regulated cell proliferation. The P2 receptor antagonists suramin and reactive blue-2 prevented the ATP-induced increase in proliferation and RT-PCR and Western blot analysis revealed that mRNAs of P2X(4/7) and P2Y(2) are abundant in cardiac fibroblasts. ATP increased phosphorylated PKB (Akt) and ERK1/2 levels; an effect antagonized by suramin, reactive blue-2, the PI3-kinase inhibitor, wortmannin, PKB inhibitor, API-2, and MAPK inhibitor, PD98059. These kinase inhibitors also prevented the ATP-induced increase in proliferation. In addition, ATP enhanced the progression of cells from the G0/G1 phase to the S phase by increasing the expression of proteins for cyclin D1 and cyclin E. Silencing the P2X(4/7) and P2Y(2) receptors with siRNA targeting the corresponding receptor diminished ATP-stimulated proliferation and migration of the cardiac fibroblasts.
Conclusion and implication: ATP activates P2X(4/7) and P2Y(2) receptors and up-regulates the proliferation of human cardiac fibroblasts by promoting cell cycling progression. It also increases the migration of these cells. These effects of ATP may be involved in cardiac remodelling of injured hearts.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures



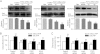

Similar articles
-
ATP stimulates mouse embryonic stem cell proliferation via protein kinase C, phosphatidylinositol 3-kinase/Akt, and mitogen-activated protein kinase signaling pathways.Stem Cells. 2006 Dec;24(12):2637-48. doi: 10.1634/stemcells.2005-0588. Epub 2006 Aug 17. Stem Cells. 2006. PMID: 16916926
-
Involvement of P2X receptors in the regulation of insulin secretion, proliferation and survival in mouse pancreatic β-cells.Cell Physiol Biochem. 2011;28(2):355-66. doi: 10.1159/000331752. Epub 2011 Aug 16. Cell Physiol Biochem. 2011. PMID: 21865744
-
Higher concentrations of extracellular ATP suppress proliferation of Caco-2 human colonic cancer cells via an unknown receptor involving PKC inhibition.Cell Physiol Biochem. 2010;26(2):125-34. doi: 10.1159/000320518. Epub 2010 Aug 24. Cell Physiol Biochem. 2010. PMID: 20798496
-
P2 purinergic receptors signal to STAT3 in astrocytes: Difference in STAT3 responses to P2Y and P2X receptor activation.Neuroscience. 2006 Oct 13;142(2):411-23. doi: 10.1016/j.neuroscience.2006.06.034. Epub 2006 Aug 14. Neuroscience. 2006. PMID: 16905269
-
Emodin Inhibits ATP-Induced Proliferation and Migration by Suppressing P2Y Receptors in Human Lung Adenocarcinoma Cells.Cell Physiol Biochem. 2017;44(4):1337-1351. doi: 10.1159/000485495. Epub 2017 Nov 29. Cell Physiol Biochem. 2017. PMID: 29183030
Cited by
-
Inferring Growth Control Mechanisms in Growing Multi-cellular Spheroids of NSCLC Cells from Spatial-Temporal Image Data.PLoS Comput Biol. 2016 Feb 11;12(2):e1004412. doi: 10.1371/journal.pcbi.1004412. eCollection 2016 Feb. PLoS Comput Biol. 2016. PMID: 26866479 Free PMC article.
-
Resolving the Ionotropic P2X4 Receptor Mystery Points Towards a New Therapeutic Target for Cardiovascular Diseases.Int J Mol Sci. 2020 Jul 15;21(14):5005. doi: 10.3390/ijms21145005. Int J Mol Sci. 2020. PMID: 32679900 Free PMC article. Review.
-
Array data extractor (ADE): a LabVIEW program to extract and merge gene array data.BMC Res Notes. 2013 Dec 1;6:496. doi: 10.1186/1756-0500-6-496. BMC Res Notes. 2013. PMID: 24289243 Free PMC article.
-
HIF-1α contributes to tube malformation of human lymphatic endothelial cells by upregulating VEGFR-3.Int J Oncol. 2019 Jan;54(1):139-151. doi: 10.3892/ijo.2018.4623. Epub 2018 Nov 5. Int J Oncol. 2019. PMID: 30431105 Free PMC article.
-
Shock wave treatment enhances cell proliferation and improves wound healing by ATP release-coupled extracellular signal-regulated kinase (ERK) activation.J Biol Chem. 2014 Sep 26;289(39):27090-27104. doi: 10.1074/jbc.M114.580936. Epub 2014 Aug 12. J Biol Chem. 2014. PMID: 25118288 Free PMC article. Clinical Trial.
References
-
- Ataullakhanov FI, Vitvitsky VM. What determines the intracellular ATP concentration. Biosci Rep. 2002;22:501–511. - PubMed
-
- Bogdanov Y, Rubino A, Burnstock G. Characterisation of subtypes of the P2X and P2Y families of ATP receptors in the foetal human heart. Life Sci. 1998;62:697–703. - PubMed
-
- Brilla CG, Maisch B, Weber KT. Myocardial collagen matrix remodelling in arterial hypertension. Eur Heart J. 1992;13(Suppl. D):24–32. - PubMed
-
- Buolamwini JK. Cell cycle molecular targets in novel anticancer drug discovery. Curr Pharm Des. 2000;6:379–392. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

