Monogenic autoimmunity
- PMID: 22224765
- PMCID: PMC6249029
- DOI: 10.1146/annurev-immunol-020711-074953
Monogenic autoimmunity
Abstract
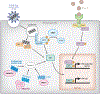
Monogenic autoimmune syndromes provide a rare yet powerful glimpse into the fundamental mechanisms of immunologic tolerance. Such syndromes reveal not only the contribution of an individual breakpoint in tolerance but also patterns in the pathogenesis of autoimmunity. Disturbances in innate immunity, a system built for ubiquitous sensing of danger signals, tend to generate systemic autoimmunity. For example, defects in the clearance of self-antigens and chronic stimulation of type 1 interferons lead to the systemic autoimmunity seen in C1q deficiency, SPENCDI, and AGS. In contrast, disturbances of adaptive immunity, which is built for antigen specificity, tend to produce organ-specific autoimmunity. Thus, the loss of lymphocyte homeostasis, whether through defects in apoptosis, suppression, or negative selection, leads to organ-specific autoimmunity in ALPS, IPEX, and APS1. We discuss the unique mechanisms of disease in these prominent syndromes as well as how they contribute to the spectrum of organ-specific or systemic autoimmunity. The continued study of rare variants in autoimmune disease will inform future investigations and treatments directed at rare and common autoimmune diseases alike.
Figures

References
-
- Berkel AI, Sanal O, Thesen R, Loos M. 1977. A case of selective C1q deficiency. Turk. J. Pediatr. 19:101–8 - PubMed
-
- Schejbel L, Skattum L, Hagelberg S, Ahlin A, Schiller B, et al. 2011. Molecular basis of hereditary C1q deficiency—revisited: identification of several novel disease-causing mutations. GenesImmun. 12:626–34 - PubMed
-
- Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. 2000. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv. Immunol. 76:227–324 - PubMed
-
- Vassallo G, Newton RW, Chieng SE, Haeney MR, Shabani A, Arkwright PD. 2007. Clinical variability and characteristic autoantibody profile in primary C1q complement deficiency. Rheumatology 46:1612–14 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

